Abstract
Biochemical properties of a putative thermostable dextranase gene from Thermotoga lettingae TMO were determined in a recombinant protein (TLDex) expressed in Escherichia coli and purified to sevenfold apparent homogeneity. The 64-kDa protein displayed maximum activity at pH 4.3, and enzyme activity was stable from pH 4.3–10. The optimal temperature was 55–60°C during 15 min incubation, and the half-life of the enzyme was 1.5 h at 65°C. The enzyme showed higher activity against α-(1 → 6) glucan and released isomaltose and isomaltotriose as main products from dextran T2000. An unusual kinetic feature of TLDex was the negative cooperative behavior on the reaction of dextran T2000 cleavage. Enzyme activity was not significantly affected by the presence of metal ions, except for the strong inhibited by 1 mM Fe2+ and Ag2+. TLDex may prove useful as an enzyme for high temperature sugar milling processes.






Similar content being viewed by others
References
Altschul SG, Madden TL, Schaffer AA, Zhang J, Shang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLSAT: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
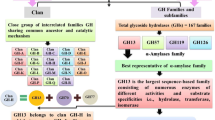
Aoki H, Sakano Y (1997) A classification of dextran-hydrolysing enzymes based on amino-acid-sequence similarities. Biochem J 323:859–861
Arnold WN, Nguyen TB, Mann LC (1998) Purification and characterization of a dextranase from Sporothrix schenckii. Arch Microbiol 170:91–98
Balk M, Weijma J, Stams AJM (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reaction. Int J Syst Evol Microbiol 52:1361–1368
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Bounias M (1980) N-(1-Naphthyl)ethylenediamine dihydrochloride as a new reagent for nanomole quantification of sugars on thin-layer plates by a mathematical calibration process. Anal Biochem 106:291–295
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britton HTS, Robinson RA (1931) Universal buffer solutions and the dissociation constant of Veronal. J Chem Soc 1:1456–1462
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Finnegan PM, Brumbley SM, Shea MGO, Nevalainen KMH, Bergquist PL (2004a) Paenibacillus isolates possess diverse dextran-degrading enzymes. J Appl Microbiol 97:477–485
Finnegan PM, Brumbley SM, Shea MGO, Nevalainen KMH, Bergquist PL (2004b) Isolation and characterization of genes encoding thermoactive and thermostable dextranases from two thermotolerant soil bacteria. Curr Microbiol 49:327–333
Finnegan PM, Brumbley SM, Shea MGO, Nevalainen KMH, Bergquist PL (2005) Diverse dextranase genes from Paenibacillus species. Arch Microbiol 183:140–147
Hild E, Brumbley SM, O’Shea MG, Nevalainen H, Bergquist QL (2007) A Paenibacillus sp. dextranase mutant pool with improved thermostability and activity. Appl Microbiol Biotechnol 75:1071–1078
Hoster F, Daniel R, Gottschalk G (2001) Isolation of a new Thermoanaerobacterium thermosaccharolyticum strain (FH1) producing a thermostable dextranase. J Gen Appl Microbiol 47:187–192
Igarashi T, Yamamoto A, Goto N (1995a) Characterization of the dextranase gene (dex) of Streptococcus mutans and its recombinant product in an Escherichia coli host. Microbiol Immunol 39:387–391
Igarashi T, Yamamoto A, Goto N (1995b) Sequence analysis of the Streptococcus mutans Ingbritt dex a gene encoding extra cellular dextranase. Microbiol Immunol 39:853–860
Igarashi T, Yamamoto A, Goto N (2000) Nucleotide sequence and molecular characterization of a dextranase gene from Streptococcus doweni. Microbiol Immunol 45:341–348
Igarashi T, Morisaki H, Goto N (2004) Molecular characterization of dextranase from Streptococcus rattus. Microbiol Immunol 48:155–162
Khalikova E, Susi P, Korpela T (2005) Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol Mol Biol Rev 69:306–325
Lawman P, Bleiweis AS (1991) Molecular cloning of the extracellular endodextranase of Streptococcus salivarius. J Bacteriol 173:7423–7428
Lee JH, Kim GH, Kim SH, Cho DL, Kim DW, Day DF, Kim D (2006) Treatment with glucanhydrolase from Lipomyces starkeyi for removal of soluble polysaccharides in sugar processing. J Microbiol Biotechnol 16:983–987
McFeeter RF (1980) A manual method for reducing sugar determinations with 2,2′-bicinchoninate reagent. Anal Biochem 103:302–306
Nakai H, Kanehisa M (1991) Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 11:95–110
Nielsen H, Engelbrecht J, Brunak S, Von Heijine G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Oguma T, Kurokawa T, Tobe K, Kobayashi M (1995) Cloning and sequence analysis of the cycloisomaltooligosaccharide glucanotransferase gene from Bacillus circulans T-3040 and expression in Escherichia coli cells. J Appl Glycosci 42:415–419
Wongchawalit J, Yamamoto T, Nakai H, Kim YM, Sato N, Nishimoto M, Okuyama M, Mori H, Saji O, Chanchao C, Wongsiri S, Surarit R, Svasti J, Chiba S, Kimura A (2006) Purification and characterization of α-glucosidase I from Japanese honeybee (Apis cerana japonica) and molecular cloning of its cDNA. Biosci Biotechnol Biochem 70:2889–2898
Wynter CVA, Galea CF, Cox LM, Dawson MW, Patel BK, Inkerman PA, Hamilton S (1995) Thermostable dextranases: screening, detection and preliminary characterization. J Appl Bacteriol 79:203–212
Wynter CVA, Chan M, De Jersey J, Patel B, Inkerman PA, Hamilton S (1997) Isolation and characterization of a thermostable dextranase. Enzyme Microb Technol 20:242–247
Yamamoto T, Terasawa K, Kim YM, Kimura A, Kitamura Y, Kobayashi M, Funane K (2006) Identification of catalytic amino acids of cyclodextran glucanotransferase from Bacillus circulans T-3040. Biosci Biotechnol Biochem 70:147–1953
Acknowledgements
This work was partially supported by grant No. RTI05-01-01 from the Regional Technology Innovation Program of the Ministry of Knowledge Economy (MKE), South Korea. We are also thankful to Korea Basic Science Institute Gwangju Branch for the DNA sequencing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YM., Kim, D. Characterization of novel thermostable dextranase from Thermotoga lettingae TMO. Appl Microbiol Biotechnol 85, 581–587 (2010). https://doi.org/10.1007/s00253-009-2121-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2121-6