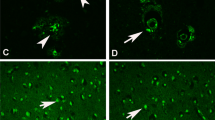
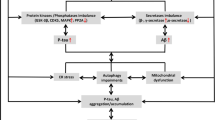
Abstract
Biometals such as copper and zinc have an important role in Alzheimer’s disease (AD). Accumulating evidence indicates that copper homeostasis is altered in AD brain with elevated extracellular and low intracellular copper levels. Studies in animals and cell cultures have suggested that increasing intracellular copper can ameliorate AD-like pathology including amyloid deposition and tau phosphorylation. Modulating copper homeostasis can also improve cognitive function in animal models of AD. Treatments are now being developed that may result in redistribution of copper within the brain. Metal ligands such as clioquinol (CQ), DP-109 or pyrrolidine dithiocarbamate (PDTC) have shown promising results in animal models of AD, however, the actual mode of action in vivo has not been fully determined. We previously reported that CQ-metal complexes were able to increase intracellular copper levels in vitro. This resulted in stimulation of phosphoinositol-3-kinase activity and mitogen activated protein kinases (MAPK). Increased kinase activity resulted in up-regulated matrix metalloprotease (MMP2 and MMP3) activity resulting in enhanced degradation of secreted Aβ. These findings are consistent with previous studies reporting metal-mediated activation of MAPKs and MMPs. How this activation occurs is unknown but evidence suggests that copper may be able to activate membrane receptors such as the epidermal growth factor receptor (EGFR) and result in downstream activation of MAPK pathways. This has been supported by studies showing metal-mediated activation of EGFR through ligand-independent processes in a number of cell-types. Our initial studies reveal that copper complexes can in fact activate EGFR. However, further studies are necessary to determine if metal complexes such as CQ-copper induce up-regulation of Aβ-degrading MMP activity through this mechanism. Elucidation of this pathway may have important implications for the development of metal ligand based therapeutics for treatment of AD and other neurodegenerative disorders.

Similar content being viewed by others
References
Abe K, Saito H (1992) Epidermal growth factor selectively enhances NMDA receptor-mediated increase of intracellular Ca2+ concentration in rat hippocampal neurons. Brain Res 587:102–108
Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, Stetler-Stevenson WG, Rosenberg GA (2004) Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke 35:e159–e162
Adamson IY, Vincent R, Bakowska J (2003) Differential production of metalloproteinases after instilling various urban air particle samples to rat lung. Exp Lung Res 29:375–388
Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732
Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI (2000) Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem 75:1219–1233
Backstrom JR, Lim GP, Cullen MJ, Tokes ZA (1996) Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40). J Neurosci 16:7910–7919
Barnham KJ, Ciccotosto GD, Tickler AK, Ali FE, Smith DG, Williamson NA, Lam YH, Carrington D, Tew D, Kocak G, Volitakis I, Separovic F, Barrow CJ, Wade JD, Masters CL, Cherny RA, Curtain CC, Bush AI, Cappai R (2003) Neurotoxic, redox-competent Alzheimer’s beta-amyloid is released from lipid membrane by methionine oxidation. J Biol Chem 278:42959–42965
Barnham KJ, Haeffner F, Ciccotosto GD, Curtain CC, Tew D, Mavros C, Beyreuther K, Carrington D, Masters CL, Cherny RA, Cappai R, Bush AI (2004a) Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer’s disease beta-amyloid. FASEB J 18:1427–1429
Barnham KJ, Masters CL, Bush AI (2004b) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214
Bayer TA, Schafer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schussel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G (2003) Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA 100:14187–14192
Behl C, Davis JB, Lesley R, Schubert D (1994) Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77:817–827
Beuche W, Yushchenko M, Mader M, Maliszewska M, Felgenhauer K, Weber F (2000) Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport 11:3419–3422
Borchardt T, Camakaris J, Cappai R, Masters CL, Beyreuther K, Multhaup G (1999) Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion. Biochem J 344(Pt 2):461–467
Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283
Bush AI (2003) The metallobiology of Alzheimer’s disease. Trends Neurosci 26:207–214
Cameron HA, Hazel TG, McKay RD (1998) Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol 36:287–306
Caragounis A, Du T, Filiz G, Laughton KM, Volitakis I, Sharples RA, Cherny RA, Masters CL, Hill AF, Li QX, Crouch PJ, Barnham KJ, White AR (2007) Differential modulation of Alzheimer’s disease amyloid beta peptide accumulation by diverse classes of metal ligands. Biochem J (in press)
Carson JA, Turner AJ (2002) Beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases?. J Neurochem 81:1–8
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Treatment with a copper–zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676
Cherny RA, Legg JT, McLean CA, Fairlie DP, Huang X, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI (1999) Aqueous dissolution of Alzheimer’s disease Abeta amyloid deposits by biometal depletion. J Biol Chem 274:23223–23228
Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM (2001) Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology 57:260–264
Crouch PJ, Barnham KJ, Bush AI, White AR (2006) Therapeutic treatments for Alzheimer’s disease based on metal bioavailability. Drug News Perspect 19:469–474
Cuajungco MP, Faget KY, Huang X, Tanzi RE, Bush AI (2000) Metal chelation as a potential therapy for Alzheimer’s disease. Ann N Y Acad Sci 920:292–304
Deibel MA, Ehmann WD, Markesbery WR (1996) Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci 143:137–142
Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, Moore A, Schlageter N, Larson S, Rapoport SI (1986) Positron emission tomography in Alzheimer’s disease. Neurology 36:879–887
Earp HS, Dawson TL, Li X, Yu H (1995) Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat 35:115–132
Eckman EA, Eckman CB (2005) Abeta-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans 33:p1101–1105
Fisk L, Nalivaeva NN, Boyle JP, Peers CS, Turner AJ (2007) Effects of hypoxia and oxidative stress on expression of neprilysin in human neuroblastoma cells and rat cortical neurones and astrocytes. Neurochem Res 32:1741–1748
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Hackel PO, Zwick E, Prenzel N, Ullrich A (1999) Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol 11:184–189
Harkness KA, Adamson P, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN (2000) Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain 123:698–709
Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI (1999) The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38:7609–7616
Huang X, Atwood CS, Moir RD, Hartshorn MA, Tanzi RE, Bush AI (2004) Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Abeta peptides. J Biol Inorg Chem 9:954–960
Kubiatowski T, Jang T, Lachyankar MB, Salmonsen R, Nabi RR, Quesenberry PJ, Litofsky NS, Ross AH, Recht LD (2001) Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J Neurosurg 95:480–488
Lee JY, Friedman JE, Angel I, Kozak A, Koh JY (2004) The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging 25:1315–1321
Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF (2003) Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem Int 43:191–196
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158:47–52
Malm TM, Iivonen H, Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Kanninen K, Salminen A, Auriola S, Van Groen T, Tanila H, Koistinaho J (2007) Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden. J Neurosci 27:3712–3721
Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. Embo J 4:2757–2763
Matsumoto K, Minamitani T, Orba Y, Sato M, Sawa H, Ariga H (2004) Induction of matrix metalloproteinase-2 by tenascin-X deficiency is mediated through the c-Jun N-terminal kinase and protein tyrosine kinase phosphorylation pathway. Exp Cell Res 297:404–414
Maurer I, Zierz S, Moller HJ (2000) A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging 21:455–462
Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX (2005) Metals and amyloid-beta in Alzheimer’s disease. Int J Exp Pathol 86:147–159
Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW (1999) Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci 19:8464–8475
Omar RA, Chyan YJ, Andorn AC, Poeggeler B, Robakis NK, Pappolla MA (1999) Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J Alzheimers Dis 1:139–145
Pawson T (1995) Protein modules and signalling networks. Nature 373:573–580
Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, Horne P, Yang J, Sekoulidis J, Coomaraswamy J, Chishti MA, Cox DW, Mathews PM, Nixon RA, Carlson GA, St George-Hyslop P, Westaway D (2003) In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci USA 100:14193–14198
Rapala-Kozik M, Kozik A, Travis J (1998) Effect of oxidation of beta-amyloid precursor protein on its beta-secretase cleavage. A model study with synthetic peptides and candidate beta-secretases. J Pept Res 52:315–320
Reeves PG, Noordewier B, Saari JT (1990) Effect of copper deficiency and cis-diamminedichloroplatinum (II) treatment on the activities of renal microvillar enzymes in rats. J Trace Elem Electrolytes Health Dis 4:11–19
Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL (2003) Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60:1685–1691
Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R (1994) Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem Biophys Res Commun 205:1755–1761
Samet JM, Dewar BJ, Wu W, Graves LM (2003) Mechanisms of Zn(2+)-induced signal initiation through the epidermal growth factor receptor. Toxicol Appl Pharmacol 191:86–93
Schlessinger J (2002) Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110:669–672
Schlessinger J (1994) SH2/SH3 signaling proteins. Curr Opin Genet Dev 4:25–30
Schlessinger J, Ullrich A (1992) Growth factor signaling by receptor tyrosine kinases. Neuron 9:383–391
Wand GS, May C, May V, Whitehouse PJ, Rapoport SI, Eipper BA (1987) Alzheimer’s disease: low levels of peptide alpha-amidation activity in brain and CSF. Neurology 37:1057–1061
White AR, Barnham KJ, Bush AI (2006a) Metal homeostasis in Alzheimer’s disease. Expert Rev Neurother 6:711–722
White AR, Du T, Laughton KM, Volitakis I, Sharples RA, Xilinas ME, Hoke DE, Holsinger RM, Evin G, Cherny RA, Hill AF, Barnham KJ, Li QX, Bush AI, Masters CL (2006b) Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J Biol Chem 281:17670–17680
Wong RW (2003) Transgenic and knockout mice for deciphering the roles of EGFR ligands. Cell Mol Life Sci 60:113–118
Wu W, Samet JM, Silbajoris R, Dailey LA, Sheppard D, Bromberg PA, Graves LM (2004) Heparin-binding epidermal growth factor cleavage mediates zinc-induced epidermal growth factor receptor phosphorylation. Am J Respir Cell Mol Biol 30:540–547
Wu W, Wang X, Zhang W, Reed W, Samet JM, Whang YE, Ghio AJ (2003) Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J Biol Chem 278:28258–28263
Yarden Y (2001) The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer 37(Suppl 4):S3–S8
Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR (1998) Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 21:75–80
Author information
Authors and Affiliations
Corresponding author
Additional information
Australian Society for Biophysics Special Issue: Metals and Membranes in Neuroscience, held in Melbourne on 11 July 2007.
Rights and permissions
About this article
Cite this article
Filiz, G., Price, K.A., Caragounis, A. et al. The role of metals in modulating metalloprotease activity in the AD brain. Eur Biophys J 37, 315–321 (2008). https://doi.org/10.1007/s00249-007-0244-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-007-0244-1