Abstract
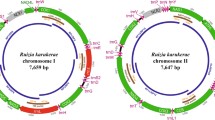
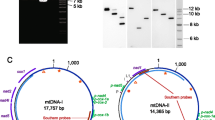
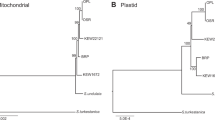
We sequenced four mitochondrial subgenomes from the potato cyst nematode Globodera pallida, previously characterized as one of the few animals to have a multipartite mitochondrial genome. The sequence data indicate that three of these subgenomic mitochondrial circles are mosaics, comprising long, multigenic fragments derived from fragments of the other circles. This pattern is consistent with the operation of intermitochondrial recombination, a process generally considered absent in animal mitochondria. We also report that many of the duplicated genes contain deleterious mutations, ones likely to render the gene nonfunctional; gene conversion does not appear to be homogenizing the different gene copies. The proposed nonfunctional copies are clustered on particular circles, whereas copies that are likely to code functional gene products are clustered on others.




Similar content being viewed by others
References
Armstrong MR, Blok VC, Phillips MS (2000) A multipartite mitochondrial genome in the potato cyst nematode Globodera pallida. Genetics 154:181–192
Awata H, Noto T, Endoh H (2005) Differentiation of somatic mitochondria and the structural changes in mtDNA during development of the dicyemid Dicyema japonicum (Mesozoa). Mol Gen Genomics 273:441–449
Coulier F, Popovici C, Villet R, Birnbaum D (2000) MetaHox gene clusters. J Exp Zool 288:345–351
Dong F, Wilson K, Makaroff C (1998) Analysis of the four cox2 genes found in turnip (Brassica campestris, Brassicaceae) mitochondria. Am J Bot 85:153–161
Dowton M (1999) Relationships among the cyclostome braconid (Hymenoptera: Braconidae) subfamilies inferred from a mitochondrial tRNA gene rearrangement. Mol Phylogenet Evol 11:283–287
Dowton M, Austin AD (1999) Evolutionary dynamics of a mitochondrial rearrangement “hotspot” in the Hymenoptera. Mol Biol. Evol 16:298–309
Dowton M, Campbell NJH (2001) Intramitochondrial recombination—Is it why some mitochondrial genes sleep around? Trends Ecol Evol 16:269–271
Dowton M, Castro LR, Austin AD (2002) Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: the examination of genome ‘morphology.’ Invert Syst 16:345–356
Dowton M, Castro LR, Campbell SL, Bargon SD, Austin AD (2003) Frequent mitochondrial gene rearrangements at the hymenopteran nad3-nad5 junction. J Mol Evol 56:517–526
Eberhard JR, Wright TF, Bermingham E (2001) Duplication and concerted evolution of the mitochondrial control region in the parrot genus Amazona. Mol Biol Evol 18:1330–1342
Eyre-Walker A, Awadalla P (2001) Does human mtDNA recombine? J Mol Evol 53:430–435
Fauron C, Casper M, Gao Y, Moore B (1995) The maize mitochondrial genome: dynamic, yet functional. Trends Genet 11:228–235
Hagelberg E (2003) Recombination or mutation rate heterogeneity? Implications for mitochondrial Eve. Trends Genet 19:84–90
Hagelberg E, Goldman N, Lió P, Whelan S, Schiefenhövel W, Clegg JB, Bowden DK (1999) Evidence for mitochodrial DNA recombination in a human population of island Melanesia. Proc R Soc Lond B 266:485–492
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hey J (2000) Human mitochondrial DNA recombination: can it be true? Trends Ecol Evol 15:181–182
Innan H, Nordberg M (2002) Recombination or mutational hot spots in human mtDNA? Mol Biol Evol 19:122–1127
Kajander OA, Rovio AT, Majamaa K, Poulton J, Spelbrink JN, Holt IJ, Karhunen PJ, Jacobs HT (2000) Human mtDNA sublimons resemble rearranged mitochondrial genoms found in pathological states. Hum Mol Genet 9:2821–2835
Kajander OA, Karhunen PJ, Holt IJ, Jacobs HT (2001) Prominent mitochondrial DNA recombination intermediates in human heart muscle. EMBO Reports 2:1007–1012
Kraytsberg Y, Schwartz M, Brown TA, Ebralidse K, Kunz WS, Clayton DA, Vissing J, Khrapko K (2004) Recombination of human mitochondrial DNA. Science 304:981
Kumazawa Y, Ota H, Nishida M, Ozawa T (1996) Gene rearrangements in snake mitochondrial genomes: highly concerted evolution of control-region-like sequences duplicated and inserted into a tRNA gene cluster. Mol Biol Evol 13:1242–1254
Ladoukakis ED, Zouros E (2001) Direct evidence for homologous recombination in mussel (Mytilus galloprovincialis) mitochondrial DNA. Mol Biol Evol 18:1168–1175
Lang BF, Gray MW, Burger G (1999) Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet 33:351–397
Levings CS 3rd, Brown GG (1989) Molecular biology of plant mitochondria. Cell 56:171–179
Lowe TM, Eddy SR (1997) tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964
Lunt DH, Hyman BC (1997) Animal mitochondrial DNA recombination. Nature 387:247
Mabuchi K, Miya M, Satoh TP, Westneat MW, Nishida M (2004) Gene rearrangement and evolution of tRNA pseudogenes in the mitochondrial genome of the parrotfish (Teleostei: Perciformes: Scaridae). J Mol Evol 59:287–297
Macey JR, Larson A, Ananjeva NB, Fang Z, Papenfuss TJ (1997) Two novel gene orders and the role of light-strand replication in rearrangement of the vertebrate mitochondrial genome. Mol Biol Evol 14:91–104
Macey JR, Papenfuss TJ, Kuehl JV, Fourcade HM, Boore JL (2004) Phylogenetic relationships among amphisbaenian reptiles based on complete mitochondrial genomic sequences. Mol Phylogenet Evol 33:22–31
Marienfeld JR, Unseld M, Brandt P, Brennicke A (1997) Mosaic open reading frames in the Arabidopsis thaliana mitochondrial genome. Biol Chem 378:859–862
Maynard Smith J, Smith NH (2002) Recombination in animal mitochondrial DNA. Mol Biol Evol 19:2330–2332
Moritz C, Dowling TE, Brown WM (1987) Evolution of animal mitochondrial DNA: relevance for population biology and systematics. Annu Rev Ecol Syst 18:269–292
Mueller RL, Boore JL (2005) Molecular mechanisms of extensive mitochondrial gene rearrangement in plethodontid salamanders. Mol Biol Evol 22:2104–2112
Pääbo S, Irwin D, Wilson A (1990) DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem 265:4718–4721
Posada D (2002) Evaluation of methods for detecting recombination from DNA sequences: empirical data. Mol Biol Evol 19:708–717
Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: Computer simulations. Proc Natl Acad Sci USA 98:13757–13762
Schon EA (2000) Mitochondrial genetics and disease. TIBS 25:555–560
Shao R, Mitani H, Barker SC, Takahashi M, Fukunaga M (2005) Novel mitochondrial gene content and gene arrangement indicate illegitimate inter-mtDNA recombination in the chigger mite, Leptotrombidium pallidum. J Mol Evol 60:764–773
Small ID, Isaac PG, Leaver CJ (1987) Stoichiometric differences in DNA molecules containing the atpA gene suggest mechanisms for the generation of mitochondrial genome diversity in maize. EMBO J 6:865–869
Small I, Suffolk R, Leaver CJ (1989) Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 58:69–76
Sunnucks P, Hales DF (1996) Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol Biol Evol 13:510–524
Tsang WY, Lemire BD (2002) Stable heteroplasmy but differential inheritance of a large mitochondrial DNA deletion in nematodes. Biochem Cell Biol 80:645–654
Watanabe KI, Bessho Y, Kawasaki M, Hori H (1999) Mitochondrial genes are found on minicircle DNA molecules in the mesozoan animal Dicyema. J Mol Biol 286:645–650
Wiuf C (2001) Recombination in human mitochondrial DNA? Genetics 159:749–756
Zevering CE, Moritz C, Heideman A, Strum RA (1991) Parallel origins of duplications and the formation of pseudogenes in mitochondrial DNA from parthenogenetic lizards (Heteronotia binoei; Gekkonidae). J Mol Evol 33:431–441
Zuckerman SH, Solus JF, Gillespie FP, Eisenstadt JM (1984) Retention of both parental mitochondrial DNA species in mouse-chinese hamster somatic cell hybrids. Somat Cell Mol Genet 10:85–92
Acknowledgments
This work was funded by grants from the Australian Research Council and the Scottish Executive Environment and Rural Affairs Department and by EU AIR3 CT-92-0062.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Reviewing Editor: Dr. Rafael Zardoya]
Rights and permissions
About this article
Cite this article
Gibson, T., Blok, V.C., Phillips, M.S. et al. The Mitochondrial Subgenomes of the Nematode Globodera pallida Are Mosaics: Evidence of Recombination in an Animal Mitochondrial Genome. J Mol Evol 64, 463–471 (2007). https://doi.org/10.1007/s00239-006-0187-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-006-0187-7