Abstract
Introduction
Cerebral small vessel disease (CSVD) and multiple sclerosis (MS) both harbor multiple, T2-hyperintense white matter lesions on conventional magnetic resonance imaging (MRI).We aimed to determine the microstructural changes via diffusion-weighted imaging (DWI) in normal appearing thalami. We hypothesized that the apparent diffusion coefficient (ADC) values would be different in CSVD and MS, since the extent of arterial involvement is different in these two diseases.
Methods
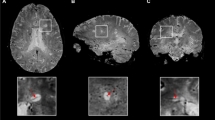
DWI was performed for 50 patients with CSVD and 35 patients with MS along with gender- and age-matched controls whose conventional MRI revealed normal findings. DWI was done with 1.5 Tesla MR devices using echo planar imaging (EPI) for b = 0, 1000 s/mm2. ADC values were obtained from the thalami which appeared normal on T2-weighted and FLAIR images. Standard oval regions of interest (ROIs) of 0.5 cm2 which were oriented parallel to the long axis of the thalamus were used for this purpose.
Results
The mean ADC value of the thalamus was (0.99 ± 0.16) × 10−3 mm2/s in patients with CSVD, whereas the mean ADC value was (0.78 ± 0.06) × 10−3 mm2/s in the control group. The mean ADC value was significantly higher in patients with CSVD compared to the controls (p < 0.001). The mean ADC values of the thalamus were (0.78 ± 0.08) × 10−3 mm2/s in MS patients, and (0.75 ± 0.08) × 10−3 mm2/s in the control group, which are not significantly different (p > 0.05).
Conclusion
Our study revealed a difference in the diffusion of the thalami between CSVD and MS. DWI may aid in the radiological disease differentiation.





Similar content being viewed by others
Abbreviations
- MS:
-
Multiple sclerosis
- CSVD:
-
Cerebral small vessel disease
- MRI:
-
Magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- ROI:
-
Region of interest
References
Bekiesińska-Figatowska M (2004) T2-hyperintense foci on brain MR imaging. Med Sci Monit 10:80–87
Wingerchuk DM, Weinshenker BG (2000) Multiple sclerosis: epidemiology, genetics, classification, natural history, and clinical outcome measures. In: Frank JA (ed) Neuroimaging clinics of North America. W.B. Saunders Company, Philadelphia, pp 611–648
Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T (2009) Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke 40(4):1195–1203
Calli C, Kitis O, Yunten N (2003) DWI findings of periventricular ischemic changes in patients with leukoaraiosis. Comput Med Imaging Graph 27:381–386
Bammer R (2003) Basic principles of diffusion-weighted imaging. Eur J Radiol 45:169–184
Helenius J, Soinne L, Salonen O, Kaste M, Tatlisumak T (2002) Leukoaraiosis, ischemic stroke, and normal white matter on diffusion-weighted MRI. Stroke 33:45–50
Eisele P, Szabo K, Griebe M, Rossmanith C, Förster A, Hennerici M, Gass A (2012) Reduced diffusion in a subset of acute MS lesions: a serial multiparametric MRI study. AJNR Am J Neuroradiol 33(7):1369–1373
Bot JC, Barkhof F, Lycklama À, Nijeholt G et al (2002) Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 223(1):46–56
Bamford J, Sandercock P, Jones L, Warlow C (1987) The natural history of lacunar infarction: the Oxfordshire Community Stroke Project. Stroke 18:545–551
O’Sullivan M (2008) Leukoaraiosis. Pract Neurol 8:26–38
Marks MP (2002) Cerebral ischemia and infarction. In: Atlas WS (ed) Magnetic resonance imaging of the brain and spine, 3rd edn. Lippincott Williams and Wilkins, Philadelphia, USA, pp 919–979
Inglese M, Park SJ, Johnson G et al (2007) Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 64:196–202
Bammer R, Fazekas F (2002) Diffusion imaging in multiple sclerosis. Neuroimaging Clin N Am 12:71–106
Filippi M, Iannucci G, Cercignani M, Assunta Rocca M, Pratesi A, Comi G (2000) A quantitative study of water diffusion in multiple sclerosis lesions and normal-appearing white matter using echo-planar imaging. Arch Neurol 57:1017–1021
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald criteria’. Ann Neurol 58(6):840–846
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Fazekas F, Barkhof F, Wahlund LO et al (2002) CT and MRI rating of white matter lesions. Cerebrovasc Dis 13:31–36
Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA (2007) Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci 257:62–66
Minagar A, Barnett MH, Benedict RHB et al (2013) The thalamus and multple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 80(2):210–219
Oliveira-Filho J, Ay H, Schaefer PW et al (2000) Diffusion-weighted magnetic resonance imaging identifies the “clinically relevant” small-penetrator infarcts. Arch Neurol 57:1009–1014
Chun T, Filippi CG, Zimmerman RD, Uluğ AM (2000) Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 21:1078–1083
Griffin CM, Chard DT, Ciccarelli O et al (2001) Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler 7:290–297
Ciccarelli O, Werring DJ, Wheeler-Kingshott CA et al (2001) Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 56:926–933
Tovar-Moll F, Evangelou TE, Chiu AW et al (2009) Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: a diffusion tensor imaging study at 3T. AJNR Am J Neuroradiol 30:1381–1387
Ceccarelli A, Rocca MA, Falini A et al (2007) Normal-appearing white and grey matter damage in MS: a volumetric and diffusion tensor MRI study at 3.0 Tesla. J Neurol 254:513–518
Hannoun S, Durand-Dubief F, Confavreux C et al (2012) Diffusion tensor-MRI evidence for extra-axonal neuronal degeneration in caudate and thalamic nuclei of patients with multiple sclerosis. AJNR Am J Neuroradiol 33(7):1363–1368
Kilsdonk ID, Wattjes MP, Lopez-Soriano A et al (2014) Improved differentiation between MS and Vascular brain lesions using FLAIR* at 7 Tesla. Eur Radiol 24(4):841–849
Sinnecker T, Dörr J, Pfueller CF et al (2012) Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology 79(7):708–714
Wuerfel J, Sinnecker T, Ringelstein EB et al (2012) Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler 18(11):1592–1599
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Ethics Committee of Cumhuriyet University School of Medicine (No. 2007-4/8; decided May 1, 2007) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öztoprak, B., Öztoprak, İ., Topalkara, K. et al. Role of thalamic diffusion for disease differentiation between multiple sclerosis and ischemic cerebral small vessel disease. Neuroradiology 57, 339–347 (2015). https://doi.org/10.1007/s00234-014-1479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1479-z