Abstract
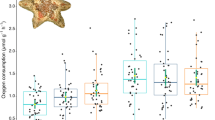
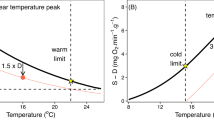
Responses by marine species to ocean acidification (OA) have recently been shown to be modulated by external factors including temperature, food supply and salinity. However the role of a fundamental biological parameter relevant to all organisms, that of body size, in governing responses to multiple stressors has been almost entirely overlooked. Recent consensus suggests allometric scaling of metabolism with body size differs between species, the commonly cited ‘universal’ mass scaling exponent (b) of ¾ representing an average of exponents that naturally vary. One model, the Metabolic-Level Boundaries hypothesis, provides a testable prediction: that b will decrease within species under increasing temperature. However, no previous studies have examined how metabolic scaling may be directly affected by OA. We acclimated a wide body-mass range of three common NE Atlantic echinoderms (the sea star Asterias rubens, the brittlestars Ophiothrix fragilis and Amphiura filiformis) to two levels of pCO2 and three temperatures, and metabolic rates were determined using closed-chamber respirometry. The results show that contrary to some models these echinoderm species possess a notable degree of stability in metabolic scaling under different abiotic conditions; the mass scaling exponent (b) varied in value between species, but not within species under different conditions. Additionally, we found no effect of OA on metabolic rates in any species. These data suggest responses to abiotic stressors are not modulated by body size in these species, as reflected in the stability of the metabolic scaling relationship. Such equivalence in response across ontogenetic size ranges has important implications for the stability of ecological food webs.


Similar content being viewed by others
References
Agutter PS, Wheatley DN (2004) Metabolic scaling: consensus or controversy? Theor Biol Med Model 1:1–13
Appelhans YS, Thomsen J, Pansch C et al (2012) Sour times: seawater acidification effects on growth, feeding behaviour and acid–base status of Asterias rubens and Carcinus maenas. Mar Ecol Prog Ser 459:85–98. doi:10.3354/meps09697
Benitez Villalobos F, Tyler PA, Young CM (2006) Temperature and pressure tolerance of embryos and larvae of the Atlantic sea stars Asterias rubens and Marthasterias glacialis (Echinodermata: Asteroidea): potential for deep-sea invasion. Mar Ecol Prog Ser 314:109–117. doi:10.3354/meps314109
Bindoff NL, Willebrand J, Artale V et al (2007) Observations: oceanic climate change and sea level. In: Climate and change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change
Bokma F (2004) Evidence against universal metabolic allometry. Funct Ecol 18:184–187. doi:10.1111/j.0269-8463.2004.00817.x
Brey T (2010) An empirical model for estimating aquatic invertebrate respiration. Methods Ecol Evol 1:92–101. doi:10.1111/j.2041-210X.2009.00008.x
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. doi:10.1890/03-9000
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Byrne M, Lamare M, Winter D et al (2013) The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae: a synthesis from the tropics to the poles. Philos Trans R Soc B Biol Sci 368:20120439. doi:10.1098/rstb.2012.0439
Carey N, Sigwart JD, Richards JG (2013) Economies of scaling: more evidence that allometry of metabolism is linked to activity, metabolic rate and habitat. J Exp Mar Bio Ecol 439:7–14. doi:10.1016/j.jembe.2012.10.013
Catarino AI, Bauwens M, Dubois P (2012) Acid–base balance and metabolic response of the sea urchin Paracentrotus lividus to different seawater pH and temperatures. Environ Sci Pollut Res Int 19:2344–2353. doi:10.1007/s11356-012-0743-1
Christensen AB, Nguyen HD, Byrne M (2011) Thermotolerance and the effects of hypercapnia on the metabolic rate of the ophiuroid Ophionereis schayeri: inferences for survivorship in a changing ocean. J Exp Mar Bio Ecol 403:31–38. doi:10.1016/j.jembe.2011.04.002
Clark MS, Husmann G, Thorne MAS et al (2013) Hypoxia impacts large adults first: consequences in a warming world. Glob Chang Biol 19:2251–2263. doi:10.1111/gcb.12197
Cohen AL, Holcomb M (2009) Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22:118–127. doi:10.5670/oceanog.2009.102
Collard M, Catarino AI, Bonnet S et al (2013) Effects of CO2-induced ocean acidification on physiological and mechanical properties of the starfish Asterias rubens. J Exp Mar Bio Ecol 446:355–362. doi:10.1016/j.jembe.2013.06.003
Darveau CA, Suarez RK, Andrews RD, Hochachka PW (2002) Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417:166–170
Dickinson GH, Matoo OB, Tourek RT et al (2013) Environmental salinity modulates the effects of elevated CO2 levels on juvenile hard-shell clams, Mercenaria mercenaria. J Exp Biol 216:2607–2618. doi:10.1242/jeb.082909
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34:1733–1743
Diffenbaugh NS, Field CB (2013) Changes in ecologically critical terrestrial climate conditions. Science 341:486–492. doi:10.1126/science.1237123
Dodds PS, Rothman DH, Weitz JS (2001) Re-examination of the “¾-law” of metabolism. J Theor Biol 209:9–27. doi:10.1006/jtbi.2000.2238
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1:169–192. doi:10.1146/annurev.marine.010908.163834
Doyle SR, Momo FR, Brêthes J-C, Ferreyra GA (2012) Metabolic rate and food availability of the Antarctic amphipod Gondogeneia antarctica (Chevreux 1906): seasonal variation in allometric scaling and temperature dependence. Polar Biol 35:413–424. doi:10.1007/s00300-011-1089-8
Duncan RP, Forsyth DM, Hone J (2007) Testing the metabolic theory of ecology: allometric scaling exponents in mammals. Ecology 88:324–333. doi:10.1890/0012-9658(2007)88[324:TTMTOE]2.0.CO;2
Dupont ST, Thorndyke MC (2012) Relationship between CO2-driven changes in extracellular acid–base balance and cellular immune response in two polar echinoderm species. J Exp Mar Bio Ecol 424–425:32–37. doi:10.1016/j.jembe.2012.05.007
Dupont ST, Thorndyke MC (2013) Direct impacts of near-future ocean acidification on sea urchins. In: Fernández-Palacios JM, de Nascimiento L, Hernández JC, et al. (eds) Climate change perspectives from the Atlantic:past, present, and future. Servicio de Publicaciones de La Universidad de La Laguna, San Cristobal de La Laguna, pp 461–485
Dupont ST, Havenhand JN, Thorndyke W et al (2008) Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Mar Ecol Prog Ser 373:285–294. doi:10.3354/meps07800
Dupont ST, Ortega-Martinez O, Thorndyke MC (2010) Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19:449–462. doi:10.1007/s10646-010-0463-6
Dupont ST, Dorey N, Stumpp M et al (2012) Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar Biol 160:1835–1843. doi:10.1007/s00227-012-1921-x
Fabry VJ, Seibel BA, Feely RA (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432. doi:10.1093/icesjms/fsn048
Forster J, Hirst AG, Atkinson D (2012) Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc Natl Acad Sci 109:19310–19314. doi:10.1073/pnas.1210460109
Gaitán-Espitia JD, Bruning A, Mondaca F, Nespolo RF (2013) Intraspecific variation in the metabolic scaling exponent in ectotherms: testing the effect of latitudinal cline, ontogeny and transgenerational change in the land snail Cornu aspersum. Comp Biochem Physiol Part A Mol Integr Physiol 165:169–177. doi:10.1016/j.cbpa.2013.03.002
Gianguzza P, Visconti G, Gianguzza F et al (2014) Temperature modulates the response of the thermophilous sea urchin Arbacia lixula early life stages to CO2-driven acidification. Mar Environ Res 93:70–77. doi:10.1016/j.marenvres.2013.07.008
Gifford ME, Clay TA, Peterman WE (2013) The effects of temperature and activity on intraspecific scaling of metabolic rates in a lungless salamander. J Exp Zool Part A Ecol Genet Physiol 319:230–236. doi:10.1002/jez.1787
Glazier DS (2005) Beyond the “¾ power law”: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev 80:611–662
Glazier DS (2006) The ¾-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 56:325–332. doi:10.1641/0006-3568(2006)56[325:TPLINU]2.0.CO;2
Glazier DS (2010) A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev 85:111–138. doi:10.1111/j.1469-185X.2009.00095.x
Hayward PJ, Ryland JS (1996) Handbook of the Marine Fauna of North-West Europe, p 800
Hemmingsen AM (1960) Energy metabolism as related to body size and respiratory surfaces, and its evolution. Steno Meml Hosp Nord Insul Laboratosium 9:6–110
Hernroth B, Baden S, Thorndyke MC, Dupont ST (2011) Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquat Toxicol 103:222–224. doi:10.1016/j.aquatox.2011.03.001
Hettinger A, Sanford E, Hill TM et al (2013) The influence of food supply on the response of Olympia oyster larvae to ocean acidification. Biogeosci Discuss 10:5781–5802. doi:10.5194/bgd-10-5781-2013
Hölker F (2003) The metabolic rate of roach in relation to body size and temperature. J Fish Biol 62:565–579. doi:10.1046/j.1095-8649.2003.00048.x
Hu MY, Casties I, Stumpp M et al (2014) Energy metabolism and regeneration impaired by seawater acidification in the infaunal brittlestar, Amphiura filiformis. J Exp Biol. doi:10.1242/jeb.100024
Hughes SJM, Ruhl HA, Hawkins LE et al (2011) Deep-sea echinoderm oxygen consumption rates and an interclass comparison of metabolic rates in Asteroidea, Crinoidea, Echinoidea, Holothuroidea and Ophiuroidea. J Exp Biol 214:2512–2521. doi:10.1242/jeb.055954
Isaac NJB, Carbone C (2010) Why are metabolic scaling exponents so controversial? Quantifying variance and testing hypotheses. Ecol Lett 13:728–735. doi:10.1111/j.1461-0248.2010.01461.x
Jensen MA, Fitzgibbon QP, Carter CG, Adams LR (2013) Effect of body mass and activity on the metabolic rate and ammonia-N excretion of the spiny lobster Sagmariasus verreauxi during ontogeny. Comp Biochem Physiol Part A Mol Integr Physiol 166:191–198. doi:10.1016/j.cbpa.2013.06.003
Kagata H, Ohgushi T (2012) Carbon to nitrogen excretion ratio in lepidopteran larvae: relative importance of ecological stoichiometry and metabolic scaling. Oikos 121:1869–1877. doi:10.1111/j.1600-0706.2012.20274.x
Kelly MW, Hofmann GE (2012) Adaptation and the physiology of ocean acidification. Funct Ecol 27:980–990. doi:10.1111/j.1365-2435.2012.02061.x
Killen SS, Atkinson D, Glazier DS (2010) The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol Lett 13:184–193. doi:10.1111/j.1461-0248.2009.01415.x
Kleiber M (1932) Body size and metabolism. Hilgardia 6:315–353
Kleiber M (1947) Body size and metabolic rate. Physiol Rev 27:511–541
Kolokotrones T, Savage VM, Deeds EJ, Fontana W (2010) Curvature in metabolic scaling. Nature 464:753–756. doi:10.1038/nature08920
Kooijman SALM (2010) Dynamic energy budget theory for metabolic organisation, Cambridge University Press, Cambridge, p 66
Kozłowski J, Konarzewski M, Gawelczyk AT (2003) Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci 100:14080–14085
Kroeker KJ, Kordas RL, Crim R et al (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol 19:1884–1896. doi:10.1111/gcb.12179
Krogh A (1916) The respiratory exchange of animals and man. Int zeits Phys chem Biol 1:173
Lawrence JM (1987) A functional biology of the echinoderms, Johns Hopkins University Press, Baltimore, p 340
Lawrence JM, Lane JM (1982) The utilization of nutrients by post-metamorphic echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 331–371
Luo YP, Wang QQ (2012) Effects of body mass and temperature on routine metabolic rate of juvenile largemouth bronze gudgeon Coreius guichenoti. J Fish Biol 80:842–851. doi:10.1111/j.1095-8649.2012.03229.x
Matozzo V, Chinellato A, Munari M et al (2012) First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS One 7:e33820. doi:10.1371/journal.pone.0033820
McElhany P, Busch DS (2013) Appropriate pCO2 treatments in ocean acidification experiments. Mar Biol 160:1807–1812. doi:10.1007/s00227-012-2052-0
McElroy DJ, Nguyen HD, Byrne M (2012) Respiratory response of the intertidal seastar Parvulastra exigua to contemporary and near-future pulses of warming and hypercapnia. J Exp Mar Bio Ecol 416–417:1–7. doi:10.1016/j.jembe.2012.02.003
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Melatunan S, Calosi P, Rundle SD et al (2011) Exposure to elevated temperature and pCO2 reduces respiration rate and energy status in the periwinkle Littorina littorea. Physiol Biochem Zool 84:583–594. doi:10.1086/662680
Morley SA, Hirse T, Pörtner HO, Peck LS (2009) Geographical variation in thermal tolerance within Southern Ocean marine ectotherms. Comp Biochem Physiol Part A Mol Integr Physiol 153:154–161
Moses ME, Hou C, Woodruff WH et al (2008) Revisiting a model of ontogenetic growth: estimating model parameters from theory and data. Am Nat 171:632–645. doi:10.1086/587073
Newell RC (1973) Factors affecting the respiration of intertidal invertebrates. Am Zool 13:513–528. doi:10.1093/icb/13.2.513
Ohlberger J, Mehner T, Staaks G, Hölker F (2012) Intraspecific temperature dependence of the scaling of metabolic rate with body mass in fishes and its ecological implications. Oikos 121:245–251. doi:10.1111/j.1600-0706.2011.19882.x
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75. doi:10.1086/282400
Pane EF, Barry JP (2007) Extracellular acid-base regulation during short-term hypercapnia is effective in a shallow-water crab, but ineffective in a deep-sea crab. Mar Ecol Prog Ser 334:1–9
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915. doi:10.1126/science.1111322
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692. doi:10.1126/science.1163156
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97. doi:10.1126/science.1135471
R Core Development Team (2013) R: a language and environment for statistical computing
Riisgård HU (1998) No foundation of a “¾ power scaling law” for respiration in biology. Ecol Lett 1:71–73. doi:10.1046/j.1461-0248.1998.00020.x
Rubner M (1883) Über den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Z Biol 19:535–562
Savage VM, Deeds EJ, Fontana W (2008) Sizing up allometric scaling theory. PLoS Comput Biol 4:e1000171. doi:10.1371/journal.pcbi.1000171
Schmidt-Nielsen K (1984) Scaling: Why is animal size so important?. Cambridge University Press, Cambridge, p 256
Seibel BA (2007) On the depth and scale of metabolic rate variation: scaling of oxygen consumption rates and enzymatic activity in the Class Cephalopoda (Mollusca). J Exp Biol 210:1–11
Seibel BA, Drazen JC (2007) The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc B Biol Sci 362:2061–2078
Sheridan JA, Bickford D (2011) Shrinking body size as an ecological response to climate change. Nat Clim Chang 1:401–406. doi:10.1038/nclimate1259
Sokal RR, Rohlf FJ (1995) Biometry, 3rd ed. 887
Stumpp M, Wren J, Melzner F et al (2011) CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comp Biochem Physiol Part A Mol Integr Physiol 160:331–340. doi:10.1073/pnas.1209174109
Stumpp M, Hu MY, Melzner F, et al. (2012) Acidified seawater impacts sea urchin larvae pH regulatory systems relevant for calcification. Proc Natl Acad Sci 109:18192–18197.doi:10.1073/pnas.1209174109
Suarez RK, Darveau CA, Childress JJ (2004) Metabolic scaling: a many-splendoured thing. Comp Biochem Physiol Part B Biochem Mol Biol 139:531–541
Symonds MRE, Elgar MA (2002) Phylogeny affects estimation of metabolic scaling in mammals. Evol Int J Org Evol 56:2330–2333
Truchot J-P, Duhamel-Jouve A (1980) Oxygen and carbon dioxide in the marine intertidal environment: diurnal and tidal changes in rockpools. Respir Physiol 39:241–254
Vaca HF, White CR (2010) Environmental modulation of metabolic allometry in ornate rainbowfish Rhadinocentrus ornatus. Biol Lett 6:136–138. doi:10.1098/rsbl.2009.0610
Waldbusser GG, Bergschneider H, Green MA (2010) Size-dependent pH effect on calcification in post-larval hard clam Mercenaria spp. Mar Ecol Prog Ser 417:171–182. doi:10.3354/meps08809
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) SMATR 3—an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259. doi:10.1111/j.2041-210X.2011.00153.x
Weldon CW, Daniels SR, Clusella-Trullas S, Chown SL (2013) Metabolic and water loss rates of two cryptic species in the African velvet worm genus Opisthopatus (Onychophora). J Comp Physiol B 183:323–332. doi:10.1007/s00360-012-0715-2
Wernberg T, Smale DA, Thomsen MS (2012) A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob Chang Biol 18:1491–1498. doi:10.1111/j.1365-2486.2012.02656.x
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126. doi:10.1126/science.276.5309.122
White CR (2011) Allometric estimation of metabolic rates in animals. Comp Biochem Physiol Part A Mol Integr Physiol 158:346–357. doi:10.1016/j.cbpa.2010.10.004
White CR, Kearney MR (2013) Determinants of inter-specific variation in basal metabolic rate. J Comp Physiol B 183:1–26. doi:10.1007/s00360-012-0676-5
White CR, Kearney MR (2014) Metabolic scaling in animals: methods, empirical results, and theoretical explanations. Compr Physiol 4:231–256. doi:10.1002/cphy.c110049
White CR, Seymour RS (2003) Mammalian basal metabolic rate is proportional to body mass 2/3. Proc Natl Acad Sci 100:4046–4049. doi:10.1073/pnas.0436428100
White CR, Phillips NF, Seymour RS (2006) The scaling and temperature dependence of vertebrate metabolism. Biol Lett 2:125–127. doi:10.1098/rsbl.2005.0378
Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: What can animal physiology tell us? J Exp Mar Bio Ecol 366:187–197. doi:10.1016/j.jembe.2008.07.024
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B Biol Sci 275:1767–1773. doi:10.1098/rspb.2008.0343
Wood HL, Spicer JI, Lowe DM, Widdicombe S (2010) Interaction of ocean acidification and temperature; the high cost of survival in the brittlestar Ophiura ophiura. Mar Biol 157:2001–2013. doi:10.1007/s00227-010-1469-6
Acknowledgments
This research was supported by the European Community—Research Infrastructure Action under the FP7 “Capacities” Programme ASSEMBLE [227799] for fieldwork and laboratory work at the Sven Lovén Centre for Marine Sciences, Kristineberg, and by the Department of Employment and Learning, N. Ireland, and Queen’s University Belfast Marine Laboratory. SD is funded by the Linnaeus Centre for Marine Evolutionary Biology at the University of Gothenburg and supported by a Linnaeus grant from the Swedish Research Councils VR and Formas. Thanks to Isabel Casties for assistance with water chemistry analysis. Three anonymous reviewers provided thoughtful and helpful suggestions that substantially improved an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.O. Pörtner.
Rights and permissions
About this article
Cite this article
Carey, N., Dupont, S., Lundve, B. et al. One size fits all: stability of metabolic scaling under warming and ocean acidification in echinoderms. Mar Biol 161, 2131–2142 (2014). https://doi.org/10.1007/s00227-014-2493-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2493-8