Abstract
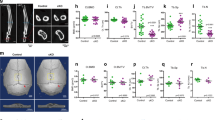
Endothelial nitric oxide synthase (eNOS) has long been held responsible for NO production by mechanically stimulated osteoblasts, but this has recently been disputed. We investigated whether one of the three known NOS isoforms is essential for NO production by mechanically stimulated osteoblasts in vitro and revisited the bone phenotype of the eNOS−/− mouse. Osteoblasts, obtained as outgrowths from mouse calvaria or long bones of wild-type (WT), eNOS−/−, inducible NOS−/− (iNOS−/−), or neuronal NOS−/− (nNOS−/−) mice, were subjected to mechanical stimulation by means of pulsating fluid flow (PFF); and NO production was determined. Tibiae and femora from 8-week-old mice were subjected to μCT and three-point bending tests. Deletion of single NOS isoforms did not lead to significant upregulation of alternate isoforms in cultured osteoblasts from WT, eNOS−/−, iNOS−/−, or nNOS−/− mice. Expression of eNOS mRNA in osteoblasts was below our detection limit, and no differences in growth between WT and eNOS−/− osteoblasts were found. PFF increased NO production by approximately fourfold in WT and eNOS−/− osteoblasts and significantly stimulated NO production in iNOS−/− and nNOS−/− osteoblasts. Tibiae and femora from WT and eNOS−/− mice showed no difference in bone volume and architecture or in mechanical parameters. Our data suggest that mechanical stimuli can enhance NO production by cultured osteoblasts singly deficient for each known NOS isoform and that lack of eNOS does not significantly affect bone mass and strength at 8 weeks of age. Our data challenge the notion that eNOS is a key effector of mechanically induced bone maintenance.





Similar content being viewed by others
References
Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408–411
van’t Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103:255–261
Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147(Suppl 1):S193–S201
Zhou L, Zhu DY (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20:223–230
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, Platts LA, Hukkanen M, Polak JM, Lanyon LE (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 14:1123–1131
Hukkanen MV, Platts LA, Fernandez DM, O’Shaughnessy M, MacIntyre I, Polak JM (1999) Developmental regulation of nitric oxide synthase expression in rat skeletal bone. J Bone Miner Res 14:868–877
Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH (1997) Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res 12:1108–1115
Loveridge N, Fletcher S, Power J, Caballero-Alias AM, Das-Gupta V, Rushton N, Parker M, Reeve J, Pitsillides AA (2002) Patterns of osteocytic endothelial nitric oxide synthase expression in the femoral neck cortex: differences between cases of intracapsular hip fracture and controls. Bone 30:866–871
van’t Hof RJ, Armour KJ, Smith LM, Armour KE, Wei XQ, Liew FY, Ralston SH (2000) Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proc Natl Acad Sci USA 97:7993–7998
Armour KE, Armour KJ, Gallagher ME, Gödecke A, Helfrich MH, Reid DM, Ralston SH (2001) Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142:760–766
Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez DM, Hukkanen M, Huang P, MacIntyre I, Polak J (2001) Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol 158:247–257
van’t Hof RJ, MacPhee J, Libouban H, Helfrich MH, Ralston SH (2004) Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology 145:5068–5074
Grassi F, Fan X, Rahnert J, Weitzmann MN, Pacifici R, Nanes MS, Rubin J (2006) Bone re/modeling is more dynamic in the endothelial nitric oxide synthase(−/−) mouse. Endocrinology 147:4392–4399
Cuzzocrea S, Mazzon E, Dugo L, Genovese T, Di PR, Ruggeri Z, Vegeto E, Caputi AP, Van De Loo FA, Puzzolo D, Maggi A (2003) Inducible nitric oxide synthase mediates bone loss in ovariectomized mice. Endocrinology 144:1098–1107
Baldik Y, Diwan AD, Appleyard RC, Fang ZM, Wang Y, Murrell GA (2005) Deletion of iNOS gene impairs mouse fracture healing. Bone 37:32–36
Watanuki M, Sakai A, Sakata T, Tsurukami H, Miwa M, Uchida Y, Watanabe K, Ikeda K, Nakamura T (2002) Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J Bone Miner Res 17:1015–1025
Sabanai K, Tsutsui M, Sakai A, Hirasawa H, Tanaka S, Nakamura E, Tanimoto A, Sasaguri Y, Ito M, Shimokawa H, Nakamura T, Yanagihara N (2008) Genetic disruption of all NO synthase isoforms enhances BMD and bone turnover in mice in vivo: involvement of the renin-angiotensin system. J Bone Miner Res 23:633–643
Skerry TM (2008) The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 473:117–123
Turner CH, Owan I, Jacob DS, McClintock R, Peacock M (1997) Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone 21:487–490
Chow JW, Fox SW, Lean JM, Chambers TJ (1998) Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res 13:1039–1044
Burger EH, Klein-Nulend J (1999) Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J 13:S101–S112
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH (1995) Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J 9:441–445
Johnson DL, McAllister TN, Frangos JA (1996) Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol Endocrinol Metab 271:E205–E208
Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH (1998) Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun 250:108–114
Das-Gupta V, Williamson RA, Pitsillides AA (2012) Expression of endothelial nitric oxide synthase protein is not necessary for mechanical strain–induced nitric oxide production by cultured osteoblasts. Osteoporos Int 23:2635–2647
Gödecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Godecke S, Schrader J (1998) Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res 82:186–194
Goodyear SR, Aspden RM (2012) Mechanical properties of bone ex vivo. Methods Mol Biol 816:555–571
Orriss IR, Taylor SE, Arnett TR (2012) Rat osteoblast cultures. Methods Mol Biol 816:31–41
Huesa C, Helfrich MH, Aspden RM (2010) Parallel-plate fluid flow systems for bone cell stimulation. J Biomech 43:1182–1189
Bakker AD, Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, Marcantonio RA, Cirelli JA, Klein-Nulend J (2009) Tumor necrosis factor alpha and interleukin-1beta modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthr Rheum 60:3336–3345
Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J (2006) Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res 21:1722–1728
Jamal SA, Hamilton CJ (2012) Nitric oxide donors for the treatment of osteoporosis. Curr Osteoporos Rep 10:86–92
Yan Q, Feng Q, Beier F (2010) Endothelial nitric oxide synthase deficiency in mice results in reduced chondrocyte proliferation and endochondral bone growth. Arthr Rheum 62:2013–2022
Yan Q, Feng Q, Beier F (2012) Reduced chondrocyte proliferation, earlier cell cycle exit and increased apoptosis in neuronal nitric oxide synthase–deficient mice. Osteoarthr Cartil 20:144–151
Fox SW, Chow JW (1998) Nitric oxide synthase expression in bone cells. Bone 23:1–6
Coers W, Timens W, Kempinga C, Klok PA, Moshage H (1998) Specificity of antibodies to nitric oxide synthase isoforms in human, guinea pig, rat, and mouse tissues. J Histochem Cytochem 46:1385–1392
Rahnert J, Fan X, Case N, Murphy TC, Grassi F, Sen B, Rubin J (2008) The role of nitric oxide in the mechanical repression of RANKL in bone stromal cells. Bone 43:48–54
Zweier JL, Wang P, Samouilov A, Kuppusamy P (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1:804–809
Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R (2000) Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275:7757–7763
Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15:391–404
Huang B, Chen SC, Wang DL (2009) Shear flow increases S-nitrosylation of proteins in endothelial cells. Cardiovasc Res 83:536–546
Acknowledgments
We thank C. M. Semeins, J. M. A. Hogervorst, and L. Rose (Amsterdam) as well as P. Crombie, S. Goodyear, and K. S. Mackenzie (Aberdeen) for their expert technical assistance. A. D. B. and J. K.-N. were supported by the Research Institute MOVE of the VU University Amsterdam; C. H., R. M. A., and M. H. H. were supported by an Oliver Bird Centre grant from the Nuffield Foundation; M. H. H. and A. H. were supported by the FP6 grant ANABONOS, and R. J. H. was supported by a career establishment award from the European Calcified Tissue Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Astrid D. Bakker and Carmen Huesa contributed equally to this study.
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bakker, A.D., Huesa, C., Hughes, A. et al. Endothelial Nitric Oxide Synthase is Not Essential for Nitric Oxide Production by Osteoblasts Subjected to Fluid Shear Stress In Vitro. Calcif Tissue Int 92, 228–239 (2013). https://doi.org/10.1007/s00223-012-9670-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-012-9670-x