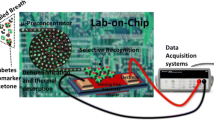
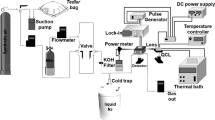
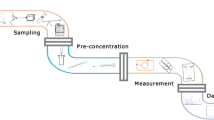
Abstract
The ammonia odor from the exhaled breath of renal patients is associated with high levels of blood urea nitrogen. Typically, in the liver, ammonia and ammonium ions are converted into urea through the urea cycle. In the case of renal dysfunction, urea is unable to be removed and that causes a buildup of excessive ammonia. As small molecules, ammonia and ammonium ions can be forced into the blood–lung barrier and occur in exhaled breath. Therefore, people with renal failure have an ammonia (fishy) odor in their exhaled breath. Thus, exhaled breath ammonia can be a potential biomarker for monitoring renal diseases during hemodialyis. In this review, we have summarized the source of ammonia in the breath of end-stage renal disease patient, cause of renal disorders, exhaled breath condensate, and breath sampling. Further, various biosensor approaches to detect exhaled ammonia from renal patients and other ammonia systems are also discussed. We conclude with future perspectives, namely colorimetric-based real-time breathing diagnosis of renal failure, which might be useful for prospective studies.


Similar content being viewed by others
References
Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55:S1–420.
Wetmore JB, Collins AJ. Global challenges posed by the growth of end-stage renal disease. Ren Replace Ther. 2016. doi:10.1186/s41100-016-0021-7.
Coresh J, Jafar TH. Disparities in worldwide treatment of kidney failure. Lancet. 2015;385:1926–8.
Shafi T, Michels WM, Levey AS, Inker LA, Dekker FW, Krediet RT, et al. Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016;89:1099–110.
Whittier WL, Sayeed K, Korbet SM. Clinical factors influencing the decision to transfuse after percutaneous native kidney biopsy. Clin Kidney J. 2016;9:102–7.
de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, Khalid T, et al. A review of the volatiles from the healthy human body. J Breath Res. 2014. doi:10.1088/1752-7155/8/1/014001.
King J, Unterkofler K, Teschl G, Teschl S, Mochalski P, Koç H, et al. A modeling-based evaluation of isothermal rebreathing for breath gas analyses of highly soluble volatile organic compounds. J Breath Res. 2012. doi:10.1088/1752-7155/6/1/016005.
Phillips M, Cataneo RN, Chaturvedi A, Kaplan PD, Libardoni M, Mundada M, et al. Detection of an extended human volatome with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. PLoS One. 2013. doi:10.1371/journal.pone.0075274.
Spanel P, Davies S, Smith D. Quantification of ammonia in human breath by the selected ion flow tube analytical method using H3O+ and O2 + precursor ions. Rapid Commun Mass Spectrom. 1998;12:763–6.
Turner C, Španěl P, Smith D. A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry, SIFT-MS. Physiol Meas. 2006;27:321–37.
Aguilar AD, Forzani ES, Nagahara LA, Amlani I, Tsui R, Tao NJ. A breath ammonia sensor based on conducting polymer nanojunctions. IEEE Sens J. 2008;8:269–73.
Hibbard T, Killard AJ. Breath ammonia analysis: clinical application and measurement. Crit Rev Anal Chem. 2011;41:21–35.
Davies S, Spanel P, Smith D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997;52:223–8.
Endre ZH, Pickering JW, Storer MK, Hu WP, Moorhead KT, Allardyce R, et al. Breath ammonia and trimethylamine allow real-time monitoring of hemodialysis efficacy. Physiol Meas. 2010;32:115–30.
Ruzsanyi V, Baumbach JI, Sielemann S, Litterst P, Westhoff M, Freitag L. Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J Chromatogr A. 2005;1084:145–51.
Ishida H, Satou T, Tsuji K, Kawashima N, Takemura H, Kosaki Y, et al. The breath ammonia measurement of the hemodialysis with a QCM-NH3 sensor. Biomed Mater Eng. 2008;18:99–106.
Norman M, Spirig C, Wolff V, Trebs I, Flechard C, Wisthaler A, et al. Intercomparison of ammonia measurement techniques at an intensively managed grassland site. Atmos Chem Phys. 2009;9:2635–45.
Wojtas TJ, Bielecki Z, Stacewicz T, Mikołajczyk J, Nowakowski M. Ultrasensitive laser spectroscopy for breath analysis. Opto-Electron Rev. 2012;20:26–39.
Smith D, Spanel P, Herbig J, Beauchamp J. Mass spectrometry for real-time quantitative breath analysis. J Breath Res. 2014. doi:10.1088/1752-7155/8/2/027101.
Righettoni M, Amann A, Pratsinis SE. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater Today. 2015;18:163–71.
Zhang L, Meng F, Chen Y, Liu J, Sun Y, Luo T, et al. A novel ammonia sensor based on high density, small diameter polypyrrole nanowire arrays. Sensors Actuators B Chem. 2009;142:204–9.
Wojkiewicz JL, Bliznyukc VN, Carquigny S, Elkamchi N, Redon N, Lasri T, et al. Nanostructured polyaniline-based composites for ppb range ammonia sensing. Sensors Actuators B Chem. 2011;160:1394–403.
Häussinger D. Ammonia, urea production, and pH regulation. The Textbook of Hepatology: from basic science to clinical practice, 3rd ed. Oxford: Wiley-Blackwell; 2007.
Narasimhan LR, Goodman W, Kumar C, Patel N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc Natl Acad Sci. 2001;98:4617–21.
Imran M, Shah Y, Nundlall S, Roberts NB, Howse M. Is blood ammonia influenced by kidney function? A prospective study. Clin Biochem. 2012;45:363–5.
Chawla LS, Egger PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66.
Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–46.
Friedman DJ, Pollak MR. Genetics of kidney failure and the evolving story of APOL1. J Clin Invest. 2011;121:3367–74.
Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest. 1980;65:1162–73.
Tizianello A, Deferrari G, Garibotto G, Robaudo C, Acquarone N, Ghiggeri GM. Renal ammoniagenesis in an early stage of metabolic acidosis in man. J Clin Invest. 1982;69:240–50.
Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–7.
Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. J Hepatol. 2003;37:233–43.
Vautz W, Nolte J, Fobbe R, Baumbach JI. Breath analysis—performance and potential of ion mobility spectrometry. J Breath Res. 2009. doi:10.1088/1752-7155/3/3/036004.
Beauchamp J. Current sampling and analysis techniques in breath research—results of a task force poll. J Breath Res. 2015. doi:10.1088/1752-7155/9/4/047107.
Beauchamp J, Herbig J, Gutmann R, Hansel A. On the use of Tedlar bags for breath-gas sampling and analysis. J Breath Res. 2008. doi:10.1088/1752-7155/2/4/046001.
Herbig J, Beauchamp J. Towards standardization in the analysis of breath gas volatiles. J Breath Res. 2014. doi:10.1088/1752-7155/8/3/037101.
Thekedar B, Szymczak W, Höllriegl V, Hoeschen C, Oeh U. Investigations on the variability of breath gas sampling using PTR-MS. J Breath Res. 2009. doi:10.1088/1752-7155/3/2/027007.
Ghimenti S, Lomonaco T, Bellagambi FG, Tabucchi S, Onor M, Trivella MG, et al. Comparison of sampling bags for the analysis of volatile organic compounds in breath. J Breath Res. 2015. doi:10.1088/1752-7155/9/4/047110.
Scott-Thomas AJ, Syhre M, Pattemore PK, Epton M, Laing R, Pearson J, et al. 2-Aminoacetophenone as a potential breath biomarker for Pseudomonas aeruginosa in the cystic fibrosis lung. BMC Pulm Med. 2010. doi:10.1186/1471-2466-10-56.
Mochalski P, Wzorek B, Sliwka I, Amann A. Suitability of different polymer bags for storage of volatile sulphur compounds relevant to breath analysis. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877:189–896.
Gilchrist FJ, Razavi C, Webb AK, Jones AM, Spaněl P, Smith D, et al. An investigation of suitable bag materials for the collection and storage of breath samples containing hydrogen cyanide. J Breath Res. 2012. doi:10.1088/1752-7155/6/3/036004.
Kang S, Paul-Thomas CL. How long may a breath sample be stored at –80 °C? A study of the stability of volatile organic compounds trapped onto a mixed Tenax: Carbograph trap adsorbent bed from exhaled breath. J Breath Res. 2016. doi:10.1088/1752-7155/10/2/026011.
Kumar S, Huang J, Abbassi-Ghadi N, Mackenzie HA, Veselkov KA, Hoare JM, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg. 2015;262:981–90.
Corradi M, Poli D, Banda I, Bonini S, Mozzoni P, Pinelli S, et al. Exhaled breath analysis in suspected cases of non-small- cell lung cancer: a cross-sectional study. J Breath Res. 2015. doi:10.1088/1752-7155/9/2/027101.
Popa C, Banita S, Patachia M, Dumitras DC. Spectroscopic study of ammonia at subjects with kidney failure: a case control study. Rev Roum Chim. 2013;58:779–84.
Hibbard T, Crowley K, Kelly F, Ward F, Holian J, Watson A, et al. Point of care monitoring of hemodialysis patients with a breath ammonia measurement device based on printed polyaniline nanoparticle sensors. Anal Chem. 2013;85:12158–65.
Wang J, Zhang W, Li L, Yu Q. Breath ammonia detection based on tunable fiber laser photoacoustic spectroscopy. Appl Phys B. 2011;103:263–9.
Konvalina G, Haick H. Sensors for breath testing: from nanomaterials to comprehensive disease detection. Acc Chem Res. 2014;47:66–676.
Nakhleh MK, Amal H, Awad H, Abu-Saleh N, Jeries R, Haick H, et al. Sensor arrays based on nanoparticles for early detection of kidney injury by breath samples. Nanomedicine Nanotechnol Biol Med. 2014;10:1767–76.
Broza YY, Haick H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine. 2013;8:785–806.
Güntner AT, Righettoni M, Pratsinis SE. Selective sensing of NH3 by Si-doped α-MoO3 for breath analysis. Sensors Actuators B Chem. 2016. doi:10.1016/j.snb.2015.09.094.
Jayasree T, Bobby M, Muttan S. Sensor data classification for renal dysfunction patients using support vector machine. J Med Biol Eng. 2015;35:759–64.
Ogimoto Y, Selyanchyn R, Takahara N, Wakamatsu S, Lee SW. Detection of ammonia in human breath using quartz crystal microbalance sensors with functionalized mesoporous SiO2 nanoparticle films. Sensors Actuators B Chem. 2015;215:428–36.
Guo D, Zhang D, Li N, Zhang L, Yang J. A novel breath analysis system based on electronic olfaction. IEEE Trans Biomed Eng. 2010;57:2753–63.
Röck F, Barsan N, Weimar U. Electronic nose: current status and future trends. Chem Rev. 2008;108:705–25.
Santini G, Mores N, Penas A, Capuano R, Mondino C, Trové A, et al. Electronic nose and exhaled breath NMR-based metabolomics applications in airways disease. Curr Top Med Chem. 2016;16:1610–30.
Güntner AT, Koren V, Chikkadi K, Righettoni M, Pratsinis SE. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 2016;1:528–35.
Hill D, Binions R. Breath analysis for medical diagnosis. Int J Smart Sens Intell Syst. 2012;5:401–40.
Kotani A, Wakabayashi Y, Kohama M, Kusu F. Determination of ammonia in exhaled breath by flow injection analysis with electrochemical detection. Electrochemistry. 2012;80:340–4.
Suslick K. An optoelectronic nose: “seeing” smells by means of colorimetric sensor arrays. MRS Bull. 2004;29:720–5.
Lim SH, Feng L, Kemling JW, Musto CJ, Suslick KS. An optoelectronic nose for the detection of toxic gases. Nat Chem. 2009;1:562–7.
Feng L, Musto CJ, Kemling JW, Lim SH, Zhong W, Suslick KS. Colorimetric sensor array for determination and identification of toxic industrial chemicals. Anal Chem. 2010;82:9433–40.
Jurmanović S, Kordić S, Steinberg MD, Steinberg IM. Organically modified silicate thin films doped with colorimetric pH indicators methyl red and bromocresol green as pH responsive sol–gel hybrid materials. Thin Solid Films. 2010;518:2234–40.
Penza M, Rossi R, Alvisi M, Signore MA, Serra E, Paolesse R, et al. Metalloporphyrins-modified carbon nanotubes networked films-based chemical sensors for enhanced gas sensitivity. Sensors Actuators B Chem. 2010;144:387–94.
Reichardt C. Solvatochromic dyes as solvent polarity indicators. Chem Rev. 1994;94:2319–58.
Mohr GJ. Chromo‐and fluororeactands: indicators for detection of neutral analytes by using reversible covalent‐bond chemistry. Chem Eur J. 2004;10:1082–90.
Greene NT, Shimizu KD. Colorimetric molecularly imprinted polymer sensor array using dye displacement. J Am Chem Soc. 2005;127:5695–700.
Kim HN, Guo Z, Zhu W, Yoon J, Tian H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem Soc Rev. 2011;40:79–93.
Kowada Y, Ozeki T, Minami T. Preparation of silica-gel film with pH indicators by the sol-gel method. J Sol-Gel Sci Technol. 2005;33:175–85.
Itagaki Y, Deki K, Nakashima SI, Sadaoka Y. Development of porphyrin dispersed sol–gel films as HCl sensitive optochemical gas sensor. Sensors Actuators B Chem. 2006;117:302–7.
Onida B, Fiorilli S, Borello L, Viscardi G, Macquarrie D, Garrone E. Mechanism of the optical response of mesoporous silica impregnated with Reichardt’s dye to NH3 and other gases. J Phys Chem B. 2004;108:16617–20.
Bang JH, Lim SH, Park E, Suslick KS. Chemically responsive nanoporous pigments: colorimetric sensor arrays and the identification of aliphatic amines. Langmuir. 2008;24:13168–72.
Askim JR, Suslick KS. Hand-held reader for colorimetric sensor arrays. Anal Chem. 2015;87:7810–6.
Suslick K, Hulkower K, Sen A, Sroka M, McNamara W (2005) Method and apparatus for detecting ammonia from exhaled breath. US Patent 20050171449A1.
Eambaipreuk A, Kladsomboon S, Kerdcharoen T (2011) Breath monitoring based on the optical electronic nose system. In: The 2011 Biomedical Engineering International Conference (BMEiCON-2011); pp. 63–66.
Devadhasan JP, Kim S. An ultrasensitive method of real time pH monitoring with complementary metal oxide semiconductor image sensor. Anal Chim Acta. 2015;858:55–9.
Devadhasan JP, Shao M, Kim S. CMOS image sensor for rapid chemical detection. J Life Sci Technol. 2014;2:20–3.
Shin W. Medical applications of breath hydrogen measurements. Anal Bioanal Chem. 2014;406:3931–9.
Ma W, Liu X, Pawliszyn J. Analysis of human breath with micro extraction techniques and continuous monitoring of carbon dioxide concentration. Anal Bioanal Chem. 2006;385:1398–408.
Sun X, Shao K, Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem. 2015;16:1–22.
Manne J, Sukhorukov O, Jager W, Tulipl J. Pulsed quantum cascade laser-based cavity ring-down spectroscopy for ammonia detection in breath. Appl Opt. 2006;45:9230–7.
van der Schee MP, Fens N, Brinkman P, Bos LD, Angelo MD, Nijsen TM, et al. Effect of transportation and storage using sorbent tubes of exhaled breath samples on diagnostic accuracy of electronic nose analysis. J Breath Res. 2013. doi:10.1088/1752-7155/7/1/016002.
Newberry C, Tierney A, Pickett-Blakely O. Lactulose hydrogen breath test result is associated with age and gender. Biomed Res Int. 2016. doi:10.1155/2016/1064029.
Kushch I, Schwarz K, Schwentner L, Baumann B, Dzien A, Schmid A, et al. Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. J Breath Res. 2008. doi:10.1088/1752-7155/2/2/026002.
Schwarz K, Pizzini A, Arendacká B, Zerlauth K, Filipiak W, Schmid A, et al. Breath acetone—aspects of normal physiology related to age and gender as determined in a PTR-MS study. J Breath Res. 2009. doi:10.1088/1752-7155/3/2/027003.
Fens N, Schee MP, Brinkman P, Sterk PJ. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin Exp Allergy. 2013;43:705–15.
Buszewski B, Ligor T, Jezierski T, Wenda-Piesik A, Walczak M, Rudnicka J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: comparison with discrimination by canines. Anal Bioanal Chem. 2012;404:141–6.
Acknowledgments
This research was supported by the R and D Program for the Society of the National Research Foundation (NRF) funded by the Ministry of Science, ICT, and Future Planning (2013M3C8A3078806 and 2015M3A9E2031372).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Krishnan, S.T., Devadhasan, J.P. & Kim, S. Recent analytical approaches to detect exhaled breath ammonia with special reference to renal patients. Anal Bioanal Chem 409, 21–31 (2017). https://doi.org/10.1007/s00216-016-9903-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9903-3