Abstract
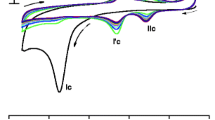
Cyclic voltammetry has been successfully used to study the oxidation of nicotinamide adenine dinucleotide (NADH) at single-wall carbon-nanotube-paste (CNTP) electrodes modified with p-methylaminophenolsulfate (p-MAP) and 3,4-dihydroxybenzaldehyde (3,4-DHB). Diffusion-like behaviour was observed for p-MAP-modified electrodes, and a diffusion coefficient of 2.4×10−6 cm2 s−1 was calculated for p-MAP in the paste. The behaviour of 3,4-DHB-modified CNTP electrodes was typical of that of surface-confined mediators. p-MAP electrocatalytic activity was first checked in solution, and a rate constant of 9.2 mol−1 L s−1 was obtained for the reaction between NADH and the mediator. The p-MAP-modified electrode did not have significant electrocatalytic activity for electro-oxidation of NADH, probably because of the formation of a complex between NADH and the confined mediator. In contrast, the 3,4-DHB-modified electrode had very good NADH electrocatalytic activity, with a heterogeneous rate constant of approximately 20×102 mol−1 L s−1.








Similar content being viewed by others
References
Moiroux J, Elving PJ (1978) Anal Chem 50:1056–1062
Jaegfeldt H (1980) J Electroanal Chem 110:295–302
Moiroux J, Elving PJ (1979) J Electroanal Chem 102:93–108
Bartlett PN, Tebbutt P, Whitaker R.G. (1991) Prog React Kinet 16:55–155
Pariente F, Tobalina F, Moreno G, Hernandez L, Lorenzo E, Abruna HD (1997) Anal Chem 69:4065–4075
Gorton L, Torstensson A, Jaegfeldt H, Johansson G (1984) J Electroanal Chem 161:103–120
Matsue T, Yamada H, Chang HC, Uchida I, Nagata K, Tomita K (1990) Biochim Biophys Acta 1038:29–38
McNeil CJ, Spoors JA, Cocco D, Cooper JM, Bannister JV (1989) Anal Chem 61:25–29
Somasundrum M, Hall J, Bannister JV (1994) Anal Chim Acta 295:47–57
Lobo MJ, Miranda AJ, Tuñón P (1997) Electroanalysis 9:191–202
Antiochia R, Cass AEG, Palleschi G (1997) Anal Chim Acta 345:17–28
Antiochia R, Lavagnini I, Magno F (1999) Electroanalysis 11:129–133
Ogino Y, Takagi K, Kano K, Ikeda T (1995) J Electroanal Chem 396:517–524
Antiochia R, Lavagnini I, Magno F (2001) Electroanalysis 13:582–586
Tse DCS, Kuwana T (1978) Anal Chem 50:1315–1318
Jaegfeldt H, Torstensson A, Gorton L, Johansson G (1981) Anal Chem 53:1979–1982
Essaadi K, Keita B, Nadjo L, Contant R (1994) J Electroanal Chem 367:275–278
Xu F, Li H, Cross SJ, Guarr TF (1994) J Electroanal Chem 368:221–225
Tortensson A, Gorton L (1981) J Electroanal Chem 130:199–207
Kulys JJ (1981) Anal Lett 14:377–381
Persson B, Gorton L (1990) J Electroanal Chem 292:115–138
Kulys J, Gleixner G, Schuhmann W, Schmidt HL (1993) Electroanalysis 5:201–207
Mano N, Kuhn A (1999) J Electroanal Chem 477:79–88
Mano N, Kuhn A (1999) Electrochem Commun 1:497–501
Mano N, Kuhn A (2001) Biosens Bioelectron 16:653–660
Munteanu FD, Mano N, Kuhn A, Gorton L (2002) Bioelectrochem 56:67–72
Persson B, Lee HS, Gorton L, Skotheim T, Bartlett P (1995) Electroanalysis 7:935–940
Gorton L (1986) Chem Soc Faraday Trans 1 82:1245–1258
Bartlett PN, Simon E, Toh CS (2002) Bioelectrochem 56:117–122
Gründig B, Wittstock G, Rudel U, Strehlitz B (1995) J Electroanal Chem 395:143–157
Prieto-Simon B, Fabregas E (2004) Biosens Bioelectron 19:1132–1138
Gorton L (1995) Electroanalysis 7:23–45
Martinez R, Ramirez MT, Gonzales I (1998) Electroanalysis 10:336–342
Malinauskas A, Ruzgas T, Gorton L (2000) J Colloid Interface Sci 224:325–332
Valentini F, Amine A, Orlanducci S, Terranova ML, Palleschi G (2003) Anal Chem 75:5413–5421
Antiochia R, Lavagnini I, Magno F, Valentini F, Palleschi G (2004) Electroanalysis 16:1451–1458
Laviron E (1979) J Electroanal Chem 101:19–28
Antiochia R, Lavagnini I, Pastore P, Magno F (2004) Bioelectrochem 64:157–163
Lavagnini I, Antiochia R, Magno F (2004) Electroanalysis 16:505–506
Nicholson RS, Shain I (1964) Anal Chem 36:706–723
Klinger RJ, Kochi K (1981) J Phys Chem 85:1731–1741
Alvarez P, Rodriguez-Granda P, Lobo-Castanon MJ, Mirando-Ordieres AJ, Tuñón-Blanco P (2003) Electrochem Comm 5:267–271
Andrieux CP, Saveant JM (1978) J Electroanal Chem 93:163–168
Acknowledgements
The financial support of Italian Ministry of Education University and Scientific Research (PRIN 2002) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antiochia, R., Lavagnini, I. & Magno, F. Electrocatalytic oxidation of NADH at single-wall carbon-nanotube-paste electrodes: kinetic considerations for use of a redox mediator in solution and dissolved in the paste. Anal Bioanal Chem 381, 1355–1361 (2005). https://doi.org/10.1007/s00216-005-3079-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3079-6