Abstract
Purpose
The aim of this study was to present and evaluate a standardized technique for brain segmentation of cranial computed tomography (CT) using probabilistic partial volume tissue maps based on a database of high resolution T1 magnetic resonance images (MRI).
Methods
Probabilistic tissue maps of white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF) were derived from 600 normal brain MRIs (3.0 Tesla, T1–3D-turbo-field-echo) of 2 large community-based population studies (BiDirect and SEARCH Health studies). After partial tissue segmentation (FAST 4.0), MR images were linearly registered to MNI-152 standard space (FLIRT 5.5) with non-linear refinement (FNIRT 1.0) to obtain non-binary probabilistic volume images for each tissue class which were subsequently used for CT segmentation. From 150 normal cerebral CT scans a customized reference image in standard space was constructed with iterative non-linear registration to MNI-152 space. The inverse warp of tissue-specific probability maps to CT space (MNI-152 to individual CT) was used to decompose a CT image into tissue specific components (GM, WM, CSF).
Results
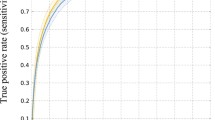
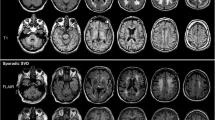
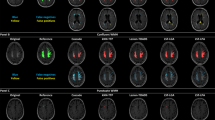
Potential benefits and utility of this novel approach with regard to unsupervised quantification of CT images and possible visual enhancement are addressed. Illustrative examples of tissue segmentation in different pathological cases including perfusion CT are presented.
Conclusion
Automated tissue segmentation of cranial CT images using highly refined tissue probability maps derived from high resolution MR images is feasible. Potential applications include automated quantification of WM in leukoaraiosis, CSF in hydrocephalic patients, GM in neurodegeneration and ischemia and perfusion maps with separate assessment of GM and WM.









Similar content being viewed by others
References
Babalola KO, Patenaude B, Aljabar P, Schnabel J, Kennedy D, Crum W, et al. Comparison and evaluation of segmentation techniques for subcortical structures in brain MRI. Med Image Comput Comput Assist Interv 2008;11(Pt 1):409–16.
Klauschen F, Goldman A, Barra V, Meyer-Lindenberg A, Lundervold A. Evaluation of automated brain MR image segmentation and volumetry methods. Hum Brain Mapp 2009;30(4):1310–27. doi:10.1002/hbm.20599.
Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi:10.1109/42.906424.
Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, et al. High-normal blood pressure is associated with poor cognitive performance. Hypertension 2008;51(3):663–8. doi:HYPERTENSIONAHA.107.105577 [pii] (10.1161/HYPERTENSIONAHA.107.105577).
Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009;46(3):786–802. doi:S1053-8119(08)01297-4 [pii] (10.1016/j.neuroimage.2008.12.037).
Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007.
Vannier MW, Butterfield RL, Jordan D, Murphy WA, Levitt RG, Gado M. Multispectral analysis of magnetic resonance images. Radiology. 1985;154(1):221–4.
Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 2005;128(Pt 6):1259–66. doi:awh508 [pii] (10.1093/brain/awh508).
Sepulcre J, Sastre-Garriga J, Cercignani M, Ingle GT, Miller DH, Thompson AJ. Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study. Arch Neurol 2006;63(8):1175–80. doi:63/8/1175 [pii] (10.1001/archneur.63.8.1175).
Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005;64(6):1032–9. doi:64/6/1032 [pii] (10.1212/01.WNL.0000154530.72969.11).
Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, et al. Tracking atrophy progression in familial Alzheimer's disease: a serial MRI study. Lancet Neurol 2006;5(10):828–34. doi:S1474-4422(06)70550-6 [pii] (10.1016/S1474-4422(06)70550-6).
Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67(4):683–6. doi:67/4/683 [pii] (10.1212/01.wnl.0000230171.23913.cf).
Henriksson KM, Wickstrom K, Maltesson N, Ericsson A, Karlsson J, Lindgren F, et al. A pilot study of facial, cranial and brain MRI morphometry in men with schizophrenia: part 2. Psychiatry Res 2006;147(2–3):187–95. doi:S0925-4927(06)00088-6 [pii] (10.1016/j.pscychresns.2006.03.004).
Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005;162(12):2233–45. doi:162/12/2233 [pii] (10.1176/appi.ajp.162.12.2233).
Strotzer M. One century of brain mapping using Brodmann areas. Klin Neuroradiol. 2009;19(3):179–86. doi:10.1007/s00062-009-9002-3.
Salomon EJ, Barfett J, Willems PW, Geibprasert S, Bacigaluppi S, Krings T. Dynamic CT angiography and CT perfusion employing a 320-detector row CT: protocol and current clinical applications. Klin Neuroradiol 2009;19(3):187–96. doi:10.1007/s00062-009-9019-7.
Pham DL, Xu C, Prince JL. Current methods in medical image segmentation. Annu Rev Biomed Eng. 2000;2:315–37. doi:2/1/315 [pii] (10.1146/annurev.bioeng.2.1.315).
Uchiyama Y, Yokoyama R, Ando H, Asano T, Kato H, Yamakawa H, et al. Computer-aided diagnosis scheme for detection of lacunar infarcts on MR images. Acad Radiol 2007;14(12):1554–61. doi:S1076-6332(07)00519-3 [pii] (10.1016/j.acra.2007.09.012).
Maillard P, Delcroix N, Crivello F, Dufouil C, Gicquel S, Joliot M, et al. An automated procedure for the assessment of white matter hyperintensities by multispectral (T1, T2, PD) MRI and an evaluation of its between-centre reproducibility based on two large community databases. Neuroradiology 2008;50(1):31–42. doi:10.1007/s00234-007-0312-3.
Dichgans M, Filippi M, Bruning R, Iannucci G, Berchtenbreiter C, Minicucci L, et al. Quantitative MRI in CADASIL: correlation with disability and cognitive performance. Neurology 1999;52(7):1361–7.
Barnes SR, Haacke EM, Ayaz M, Boikov AS, Kirsch W, Kido D. Semiautomated detection of cerebral microbleeds in magnetic resonance images. Magn Reson Imaging 2011;29(6):844–52. doi:S0730-725X(11)00097-X [pii] (10.1016/j.mri.2011.02.028).
Eckert B. Acute stroke therapy 1981–2009. Klin Neuroradiol. 2009;19(1):8–19. doi:10.1007/s00062-009-8033-0.
Kucinski T. Imaging in acute stroke—a personal view. Klin Neuroradiol. 2009;19(1):20–30. doi:10.1007/s00062-009-8030-3.
Shinar D, Gross CR, Hier DB, Caplan LR, Mohr JP, Price TR, et al. Interobserver reliability in the interpretation of computed tomographic scans of stroke patients. Arch Neurol. 1987;44(2):149–55.
Schriger DL, Kalafut M, Starkman S, Krueger M, Saver JL. Cranial computed tomography interpretation in acute stroke: physician accuracy in determining eligibility for thrombolytic therapy. JAMA 1998;279(16):1293–7. doi:joc80138 [pii].
Grotta JC, Chiu D, Lu M, Patel S, Levine SR, Tilley BC, et al. Agreement and variability in the interpretation of early CT changes in stroke patients qualifying for intravenous rtPA therapy. Stroke. 1999;30(8):1528–33.
Maldjian JA, Chalela J, Kasner SE, Liebeskind D, Detre JA. Automated CT segmentation and analysis for acute middle cerebral artery stroke. AJNR Am J Neuroradiol. 2001;22(6):1050–5.
Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, et al. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke 2011;42(5):1255–60. doi:STROKEAHA.110.600940 [pii] (10.1161/STROKEAHA.110.600940).
Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology 2008;247(3):818–25. doi:2473070551 [pii] (10.1148/radiol.2473070551).
Dijkhuizen RM, Knollema S, van der Worp HB, Ter Horst GJ, De Wildt DJ, Berkelbach van der Sprenkel JW, et al. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: evidence for region-specific sensitivity and delayed damage. Stroke. 1998;29(3):695–704.
Arakawa S, Wright PM, Koga M, Phan TG, Reutens DC, Lim I, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke 2006;37(5):1211–6. doi:01.STR.0000217258.63925.6b [pii] (10.1161/01.STR.0000217258.63925.6b).
Hu Y, Xie M, editors. Automatic Segmentation of Brain CT Image Based on Multiplicate Features and Decision Tree. International Conference on Communications, Circuits and Systems, 2007. ICCCAS 2007.; 2007 11–13 July 2007; Kokura.
Gupta V, Ambrosius W, Qian G, Blazejewska A, Kazmierski R, Urbanik A, et al. Automatic segmentation of cerebrospinal fluid, white and gray matter in unenhanced computed tomography images. Acad Radiol 2010;17(11):1350–8. doi:S1076-6332(10)00310-7 [pii] (10.1016/j.acra.2010.06.005).
Ruttimann UE, Joyce EM, Rio DE, Eckardt MJ. Fully automated segmentation of cerebrospinal fluid in computed tomography. Psychiatry Res. 1993;50(2):101–19. doi:S0165-1781(05)80005-8 [pii].
DeLeo JM, Schwartz M, Creasey H, Cutler N, Rapoport SI. Computer-assisted categorization of brain computerized tomography pixels into cerebrospinal fluid, white matter, and gray matter. Comput Biomed Res. 1985;18(1):79–88.
Lee TH, Fauzi MFA, Komiya R. Segmentation of CT brain images using K-means and EM clustering. Fifth International Conference on Computer Graphics, Imaging and Visualisation (CGIV) 2008; 399–344.
Ito H, Inoue K, Goto R, Kinomura S, Taki Y, Okada K, et al. Database of normal human cerebral blood flow measured by SPECT: I. Comparison between I-123-IMP, Tc-99m-HMPAO, and Tc-99m-ECD as referred with O-15 labeled water PET and voxel-based morphometry. Ann Nucl Med 2006;20(2):131–8.
Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 2001;356(1412):1293–322. doi:10.1098/rstb.2001.0915.
Conflict of Interest
The authors declare that there is no current or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Linear Registration
The FMRIB’s linear image registration tool (FLIRT) is part of the FMRIB software library (FSL), a comprehensive library of analysis tools for FMRI, MRI and DTI brain imaging data (http://www.fmrib.ox.ac.uk/fsl/fsl/list.html). It registers two brain volumes (an input image and reference image) by linear transformation (affine registration). The result is a spatially registered input image to the target space (reference image) with a transformation matrix containing the geometric transformation parameters. The matrix can be mathematically inverted to perform an inverse transformation (reference to input image).
Degrees of Freedom
Affine linear transformation with FLIRT can be performed with 12 DOF. This means four operations (rotation, translation, scaling, skew) are possible for each of the three dimensions x, y, z of the input image to match it with the reference image.
Non-Linear Registration
Because a linear transform is usually not sufficient to achieve good registration, the FMRIB’s non-linear image registration tool (FNIRT) is used after linear registration with FLIRT for further non-linear refinement of an input image to a reference image (http://www.fmrib.ox.ac.uk/fsl/fnirt). For instance, non-linear deformation will account for brain atrophy and enlarged ventricles of an input image image allowing a better match with the reference image. It calculates a deformation field (warp coefficients) for non-linear transformation of the pre-aligned input image to the reference image. The warp field can be inverted to perform an inverse non-linear transformation (reference to input image).
Standard Space MNI-152
A standard space is a common reference coordinate system. An input image can be registered to an appropriate reference image in standard space. A commonly used standard space is based on the MNI-152 standard brain of the Montreal Neurological Institute (MNI) which is the average of 152 normal MRI scans. There are numerous binary and probabilistic atlases of brain structures in the space defined by the MNI-152 template (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html). Atlases can be used for further automated anatomical analysis of an input image registered to standard space.
Partial Tissue Automatic Segmentation
The FMRIB’s automated segmentation tool (FAST) segments an MRI of the brain into different tissue types (GM, WM and CSF). The underlying method is based on a hidden Markov random field model and an associated expectation-maximization algorithm (http://www.fmrib.ox.ac.uk/analysis/research/fast/). The tool performs fully automated and robust probabilistic or partial volume tissue segmentations. The input (a high resolution 3D T1 MR image) is segmented with output of different tissue maps where the gray value of the voxel encodes the probability of that tissue present in that voxel.
Rights and permissions
About this article
Cite this article
Kemmling, A., Wersching, H., Berger, K. et al. Decomposing the Hounsfield Unit. Clin Neuroradiol 22, 79–91 (2012). https://doi.org/10.1007/s00062-011-0123-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-011-0123-0