Abstract
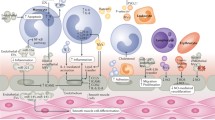
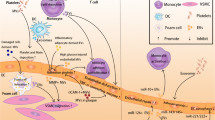
Circulating extracellular vesicles (EVs) comprise a heterogeneous population of vesicular structures. According to the current paradigm, there are three types of EVs, including exosomes, microvesicles and apoptotic bodies, that are differentiated in their size, formation, and release mechanisms. EVs were shown to act as a ‘post service’ that serves a long-distance delivery of complex cellular messages. The cargo of EVs consists of a variety of biomolecules including proteins, DNA, mRNA, and non-coding RNA. In normal or pathological conditions, EVs deliver various molecules to the recipient cells. Those molecules greatly vary depending on the microenvironmental stimuli. In proinflammatory conditions such as atherosclerosis and other cardiovascular diseases, EVs derived from vascular endothelial cells, vascular smooth muscle cells, macrophages, and other circulating immune cells mainly possess proinflammatory properties. However, the capacity of circulating EVs to stably maintain and deliver a variety of biomolecules makes these microparticles to be a promising therapeutic tool for treatment of cardiovascular pathology. To date, circulating EVs were evaluated to be as a source of valuable diagnostic and prognostic biomarkers such as microRNA. Circulating EVs keep a great therapeutic potential to serve as vehicles for targeted therapy of cardiovascular diseases.


Similar content being viewed by others
References
Chistiakov DA, Chekhonin VP (2014) Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumour Biol 35:8425–8438
Simons M, Raposo G (2009) Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–5781
Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123:1603–1611
Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C (2011) Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res 1:98–110
Castejón OJ, Arismendi GJ (2006) Nerve cell death types in the edematous human cerebral cortex. J Submicrosc Cytol Pathol 38:21–36
Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C (2009) Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2:ra81
Danielson KM, Das S (2014) Extracellular vesicles in heart disease: excitement for the future? Exosomes Microvesicles. doi:10.5772/58390
de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW (2012) Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. doi:10.3402/jev.v1i0.18396
Pallet N, Sirois I, Bell C, Hanafi LA, Hamelin K, Dieudé M, Rondeau C, Thibault P, Desjardins M, Hebert MJ (2013) A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13:1108–1120
Russell RC, Yuan HX, Guan KL (2014) Autophagy regulation by nutrient signaling. Cell Res 24:42–57
Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruïne AP, Baldwin HS, van Engeland M (2010) The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J 24:4153–4166
Ray R, Chen G, Vande Velde C, Cizeau J, Park JH, Reed JC, Gietz RD, Greenberg AH (2000) BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem 275:1439–1448
Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni M, An G, Dong M, Liu X, Zhu F, Zhang W, Gao F, Chen YH, Zhang Y (2013) Enhanced atherosclerosis in TIPE2-deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol 191:4849–4857
Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Wang X, Qian LJ (2009) Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun 387:229–233
Lancaster GI, Febbraio MA (2005) Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280:23349–23355
Chen X, Sun Z, Du X, Liu C, Liu Y, Wu L (2004) Study on the relationship between heat shock protein 70 and toll-like receptor-4 of monocytes. J Huazhong Univ Sci Technol Med Sci 24:560–562
Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39:133–144
Taïbi F, Metzinger-Le Meuth V, Massy ZA, Metzinger L (2014) miR-223: an inflammatory oncomiR enters the cardiovascular field. Biochim Biophys Acta 1842:1001–1009
Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, Naoe T (2011) Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol Ther 19:395–399
Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH (2013) Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem 288:34343–34351
Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, Gold JD, Wu JC (2014) Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation 130(11 Suppl 1):S60–S69
Boon RA, Horrevoets AJ (2009) Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie 29:39–43
Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14:249–256
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C (2009) MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 105:158–166
Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I (2013) MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 123:2143–2154
Halkein J, De Windt LJ (2013) MiR-223: sailing to terra incognita for microRNAs in platelets. Thromb Haemost 110:1112–1113
Yang Y, Rodriguez JE, Kitsis RN (2013) A microRNA links prolactin to peripartum cardiomyopathy. J Clin Invest 123:1925–1927
Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, Crosby-Nwaobi R, Prokopi M, Drozdov I, Langley SR, Sivaprasad S, Markus HS, Mitchell JA, Warner TD, Kiechl S, Mayr M (2013) Circulating microRNAs as novel biomarkers for platelet activation. Circ Res 112:595–600
van der Zee PM, Biró E, Ko Y, de Winter RJ, Hack CE, Sturk A, Nieuwland R (2006) P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem 52:657–664
Tan KT, Tayebjee MH, Lynd C, Blann AD, Lip GY (2005) Platelet microparticles and soluble P selectin in peripheral artery disease: relationship to extent of disease and platelet activation markers. Ann Med 37:61–66
Merten M, Pakala R, Thiagarajan P, Benedict CR (1999) Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb/IIIa-dependent mechanism. Circulation 99:2577–2582
Barry OP, Praticò D, Savani RC, FitzGerald GA (1998) Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest 102:136–144
Kaneider NC, Kaser A, Tilg H, Ricevuti G, Wiedermann CJ (2003) CD40 ligand-dependent maturation of human monocyte-derived dendritic cells by activated platelets. Int J Immunopathol Pharmacol 16:225–231
Sadallah S, Eken C, Martin PJ, Schifferli JA (2011) Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol 186:6543–6552
Hagihara M, Higuchi A, Tamura N, Ueda Y, Hirabayashi K, Ikeda Y, Kato S, Sakamoto S, Hotta T, Handa S, Goto S (2004) Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro. J Immunol 172:5297–5303
Sadallah S, Eken C, Schifferli JA (2011) Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol 163:26–32
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94:3791–3799
Weber A, Köppen HO, Schrör K (2000) Platelet-derived microparticles stimulate coronary artery smooth muscle cell mitogenesis by a PDGF-independent mechanism. Thromb Res 98:461–466
Liu ML, Scalia R, Mehta JL, Williams KJ (2012) Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler Thromb Vasc Biol 32:2113–2121
Park SY, Lee JH, Kim YK, Kim CD, Rhim BY, Lee WS, Hong KW (2005) Cilostazol prevents remnant lipoprotein particle-induced monocyte adhesion to endothelial cells by suppression of adhesion molecules and monocyte chemoattractant protein-1 expression via lectin-like receptor for oxidized low-density lipoprotein receptor activation. J Pharmacol Exp Ther 312:1241–1248
Chyrchel B, Totoń-Żurańska J, Kruszelnicka O, Chyrchel M, Mielecki W, Kołton-Wróż M, Wołkow P, Surdacki A (2014) Association of plasma miR-223 and platelet reactivity in patients with coronary artery disease on dual antiplatelet therapy: a preliminary report. Platelets 2014:1–5
Gatsiou A, Boeckel JN, Randriamboavonjy V, Stellos K (2012) MicroRNAs in platelet biogenesis and function: implications in vascular homeostasis and inflammation. Curr Vasc Pharmacol 10:524–531
Hulsmans M, Holvoet P (2013) MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res 100:7–18
Gidlöf O, van der Brug M, Ohman J, Gilje P, Olde B, Wahlestedt C, Erlinge D (2013) Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 121(3908–3917):S1–S26
Edelstein LC, Bray PF (2011) MicroRNAs in platelet production and activation. Blood 117:5289–5296
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104:3257–3266
Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE (2008) Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol 180:3081–3090
Segura E, Guérin C, Hogg N, Amigorena S, Théry C (2007) CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol 179:1489–1496
Biró E, Sturk-Maquelin KN, Vogel GM, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R (2003) Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost 1:2561–2568
Abid Hussein MN, Böing AN, Biró E, Hoek FJ, Vogel GM, Meuleman DG, Sturk A, Nieuwland R (2008) Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb Res 121:865–8671
Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B (2003) Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 197:1585–1598
Furie B, Furie BC (2004) Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol Med 10:171–178
Pisetsky DS (2014) The expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol Med 20:158–163
Mooberry MJ, Key NS (2015) Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry A. doi:10.1002/cyto.a.22647
Langer F, Ruf W (2014) Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost 111:590–597
Chen VM, Hogg PJ (2013) Encryption and decryption of tissue factor. J Thromb Haemost 11(Suppl 1):277–284
Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ (2006) Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry 45:12020–12028
Mallat Z, Hugel B, Ohan J, Lesèche G, Freyssinet JM, Tedgui A (1999) Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 99:348–353
Leroyer AS, Tedgui A, Boulanger CM (2008) Role of microparticles in atherothrombosis. J Intern Med 263:528–537
Macey MG, Wolf SI, Lawson C (2010) Microparticle formation after exposure of blood to activated endothelium under flow. Cytometry A 77:761–768
Date K, Hall J, Greenman J, Maraveyas A, Madden LA (2013) Tumour and microparticle tissue factor expression and cancer thrombosis. Thromb Res 131:109–115
Mezouar S, Mege D, Darbousset R, Farge D, Debourdeau P, Dignat-George F, Panicot-Dubois L, Dubois C (2014) Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin Oncol 41:346–358
Lima LG, Leal AC, Vargas G, Porto-Carreiro I, Monteiro RQ (2013) Intercellular transfer of tissue factor via the uptake of tumor-derived microvesicles. Thromb Res 132(4):450–456
Hoyer FF, Giesen MK, Nunes França C, Lütjohann D, Nickenig G, Werner N (2012) Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice. J Cell Mol Med 16:2777–2788
Mastronardi ML, Mostefai HA, Soleti R, Agouni A, Martínez MC, Andriantsitohaina R (2011) Microparticles from apoptotic monocytes enhance nitrosative stress in human endothelial cells. Fundam Clin Pharmacol 25:653–660
Basavaraj MG, Sovershaev MA, Egorina EM, Gruber FX, Bogdanov VY, Fallon JT, Østerud B, Mathiesen EB, Hansen JB (2012) Circulating monocytes mirror the imbalance in TF and TFPI expression in carotid atherosclerotic plaques with lipid-rich and calcified morphology. Thromb Res 129:e134–e141
Egorina EM, Sovershaev MA, Bogdanov VY, Sovershaev TA, Fallon JT, Seredkina N, Østerud B, Hansen JB (2011) Low thrombogenicity of calcified atherosclerotic plaques is associated with bone morphogenetic protein-2-dependent inhibition of tissue factor expression. Blood Coagul Fibrinolysis 22:642–650
Tsiantoulas D, Perkmann T, Afonyushkin T, Mangold A, Prohaska TA, Papac-Milicevic N, Millischer V, Bartel C, Horkko S, Boulanger CM, Tsimikas S, Fischer MB, Witztum JL, Lang IM, Binder CJ (2015) Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J Lipid Res 56:440–448
Soleti R, Lauret E, Andriantsitohaina R, Carmen Martínez M (2012) Internalization and induction of antioxidant messages by microvesicles contribute to the antiapoptotic effects on human endothelial cells. Free Radic Biol Med 53:2159–2170
Jaiswal R, Luk F, Gong J, Mathys JM, Grau GE, Bebawy M (2012) Microparticle conferred microRNA profiles—implications in the transfer and dominance of cancer traits. Mol Cancer 11:37
Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K (2012) Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res 93:633–644
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE (2012) Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119:756–766
Mocharla P, Briand S, Giannotti G, Dörries C, Jakob P, Paneni F, Lüscher T, Landmesser U (2013) AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood 121:226–236
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res 107:677–684
Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T (2011) Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 4:446–454
Jentzsch C, Leierseder S, Loyer X, Flohrschütz I, Sassi Y, Hartmann D, Thum T, Laggerbauer B, Engelhardt S (2012) A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol 52:13–20
Li AY, Yang Q, Yang K (2015) miR-133a mediates the hypoxia-induced apoptosis by inhibiting TAGLN2 expression in cardiac myocytes. Mol Cell Biochem 400:173–181
Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A, Blanca V, Núñez-Gil IJ, Valiente I, Ruíz-Sauri A, Sepúlveda P, Tiburcy M, Zimmermann WH, Bernad A (2014) miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep 3:1029–1042
Hua Y, Zhang Y, Ren J (2012) IGF-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: role of microRNA-1 and microRNA-133a. J Cell Mol Med 16:83–95
Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Zhang S, Hajjar RJ, Lebeche D (2013) Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc 2:e000078
Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I (2013) Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res 113:322–326
Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N (2014) MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc 3:e001249
Nazari-Jahantigh M, Egea V, Schober A, Weber C (2014) MicroRNA-specific regulatory mechanisms in atherosclerosis. J Mol Cell Cardiol. doi:10.1016/j.yjmcc.2014.10.021
Shatseva T, Lee DY, Deng Z, Yang BB (2011) MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J Cell Sci 124(Pt 16):2826–2836
Fleury A, Martinez MC, Le Lay S (2014) Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol 5:370
Loyer X, Vion AC, Tedgui A, Boulanger CM (2014) Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res 114:345–353
Chistiakov DA, Sobenin IA, Orekhov AN (2012) Strategies to deliver microRNAs as potential therapeutics in the treatment of cardiovascular pathology. Drug Deliv 19:392–405
Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A (2013) MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol 33:449–454
Ma X, Ma C, Zheng X (2013) MicroRNA-155 in the pathogenesis of atherosclerosis: a conflicting role? Heart Lung Circ 22:811–818
Zhang E, Wu Y (2014) Dual effects of miR-155 on macrophages at different stages of atherosclerosis: LDL is the key? Med Hypotheses 83:74–78
Gill R, Kuriakose R, Gertz ZM, Salloum FN, Xi L, Kukreja RC (2015) Remote ischemic preconditioning for myocardial protection: update on mechanisms and clinical relevance. Mol Cell Biochem 402:41–49
Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á, Buzás EI, Ferdinandy P (2014) Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 68:75–78
Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN (2014) MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 109:423
Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H (2015) Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 178:239–246
Feng Y, Huang W, Wani M, Yu X, Ashraf M (2014) Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 9:e88685
Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP (2007) Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1:129–137
Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DP (2011) Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 6:206–214
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4:214–222
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3 K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10:301–312
Lai RC, Yeo RW, Tan KH, Lim SK (2013) Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv 31:543–551
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65:336–341
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ (2012) Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc 7:2112–2126
Gao Y, Wang ZY, Zhang J, Zhang Y, Huo H, Wang T, Jiang T, Wang S (2014) RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain. Biomacromolecules 15:1010–1018
Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martínez MC, Andriantsitohaina R (2007) Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J 21:2735–2741
Benameur T, Soleti R, Porro C, Andriantsitohaina R, Martínez MC (2010) Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS ONE 5:e12688
Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW (2012) Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 111:312–321
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108:5003–5008
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–433
Gallo A, Tandon M, Alevizos I, Illei GG (2012) The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 7:e30679
Cheng L, Sharples RA, Scicluna BJ, Hill AF (2014) Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 26:3. doi:10.3402/jev.v3.23743
Siljander PR (2011) Platelet-derived microparticles - an updated perspective. Thromb Res 127:S30–S33
Acknowledgments
We wish to thank the Russian Scientific Foundation (Grant 14-15-00112), Russian Federation for support of our work.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chistiakov, D.A., Orekhov, A.N. & Bobryshev, Y.V. Extracellular vesicles and atherosclerotic disease. Cell. Mol. Life Sci. 72, 2697–2708 (2015). https://doi.org/10.1007/s00018-015-1906-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1906-2