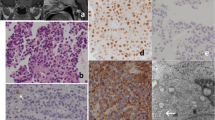
Abstract
In situ hybridization (ISH), which can manifest the specific gene expression of anterior pituitary hormones (mRNA), as well as immunohistochemistry (IHC), is needed to clarify the endocrine function of pituitary adenomas. With the aid of nonisotopic ISH, which has several advantages over isotopic ISH, we examined the expression of pituitary hormone mRNAs in 14 clinically nonfunctioning adenomas, which were considered to be a subtype of gonadotroph adenomas. Gene expression of growth hormone (GH; 4/14), prolactin (PRL; 5/14), adrenocorticotroph hormone (ACTH; 4/14), and gonadotropin were detected with our nonisotopic ISH studies. It is suggested from our ISH studies that some clinically nonfunctioning adenomas are composed of hormone (or subunit) producing cells and may be derived from plurihormonal primordial stem cells.
Similar content being viewed by others
References
Horvath E, Kovacs K. Pituitary gland. Pathol Res Pract 183: 129–142, 1988.
Kovacs K, Horvath E, Tumors of the pituitary gland. Atlas of Tumor Pathology, 2nd ser., Fascicle 21. Armed Forces Institute of Pathology, Washington, DC, 1986.
Asa SL, Gerrie BM, Singer W, Horvath E, Kovacs K. Gonadotropin secretion in vitro by human pituitary null cell adenomas and oncocytomas. J Clin Endocrinol Metab 62: 1011–1019, 1986.
Beckers A, Stevenaert A, Mashiter K, Hennen G. Follicle-stimulating hormone-secreting pituitary adenomas. J Clin Endocrinol Metab 61: 525–528, 1985.
Black PM, Hsu DW, Klibanski A, Kliman B, Jameson JL, Ridgway EC, Hedley-Whyte ET, Zervas NT. Hormone producing in clinically nonfunctioning pituitary adenomas. J Neurosurg 66: 244–250, 1987.
Mashiter K, Adam E, Van Noorden S. Secretion of LH, FSH and PRL shown by cell culture and immunocytochemistry of human functionless pituitary adenomas. Clin Endocrinol (Oxford) 15: 103–112, 1981.
Osamura RY, Watanabe K. Immunohis- tochemical studies of human FSH producing pituitary adenomas. Virchows Arch A Pathol Anat 413: 61–68, 1988.
Ridgway EC. Glycoprotein hormone produc- tion by pituitary tumors. In: Black PM, Zervas NT, Ridgway EC, Martin JB, eds. Pituitary gland. New York: Raven, 1984; 343–363.
Sanno N, Teramoto A, Inada K, Osamura RY. Immunohistochemical and clinicoendocrinological studies of gonadotropin producing pituitary adenomas. Brain Nerve (Jpn) 44: 745–753, 1992.
Snyder PJ. Gonadotroph cell adenomas of the pituitary. Endocr Rev 6: 552–563, 1985.
Surmont DW, Winslow CL, Loizou M, White MC, Adams EF, Mashiter K. Gonadotrophin and alpha subunit secretion by human functionless pituitary adenomas in cell culture: long term effects of luteinizing hormone releasing hormone and thyrotrophin releasing hormone. Clin Endocrinol (Oxford) 19: 325–336, 1983.
Takeuchi J, Handa H, Suda K, Aso T. In vitro secretion of follicle-stimulating hormone by pituitary chromophobe adenomas. Surg Neurol 14: 303–309, 1980.
Baz E, Saeger W, Uhlig H, Fehr S, Lüdecke DK. HGH, PRL, and βHCGβLH gene expression in clinically inactive pituitary adenomas detected by in situ hybridization. Virchows Arch A Pathol Anat 418: 405–410, 1991.
Hankin RC, Lloyd RV. Detection of messenger RNA in routinely processed tissue sections with biotinylated oligonucleotide probes. Am J Clin Pathol 92: 166–170, 1989.
Kilár F, Muhr C, Funa K. In situ hybridization histochemistry of mRNAs for hormones and chromogranins in normal pituitary tissue and pituitary adenoma. Acta Endocrinologica (Copenh) 125: 628–636, 1991.
Kovacs K, Lloyd RV, Horvath E, Asa SL, Stefaneanu L, Killinger DW, Smyth H. Silent somatotroph adenomas of the human pituitary. Am J Pathol 134: 345–353, 1989.
Lloyd RV. Analysis of human pituitary tumors by in situ hybridization. Pathol Res Pract 183: 558–560, 1988.
Lloyd RV, Iacangelo A, Eiden LE, Cano M, Jin L, Grimes M. Chromogranin A and B messenger ribonucleic acids in pituitary and other normal and neoplastic human endocrine tissues. Lab Invest 60: 548–556, 1989.
Lloyd RV, Cano M, Chandler WF, Barkan AL, Horvath E, Kovacs K. Human growth hormone and prolactin secreting pituitary adenomas analyzed by in situ hybridization. Am J Pathol 134: 605–613, 1989.
Lloyd RV, Jin L, Fields K. Detection of chromogranins A and B in endocrine tissues with radioactive and biotinylated oligonucleotide probes. Am J Surg Pathol 14: 35–43, 1990.
Lloyd RV, Fields K, Jin L, Horvath E, Kovacs K. Analysis of endocrine active and clinically silent corticotroph adenomas by in situ hybridization. Am J Pathol 137: 479–488, 1990.
Lloyd RV, Jin L, Chandler WF. In situ hybridization in the study of pituitary tissues. Pathol Res Pract 187: 552–555, 1991.
Lloyd RV, Jin L, Fields K, Chandler WF, Horvath E, Stefaneanu L, Kovacs K. Analysis of pituitary hormones and chromogranin A mRNAs in null cell adenomas, oncocytomas, and gonadotroph adenomas by in situ hybridization. Am J Pathol 139: 553–564, 1991.
Singer RH, Lawrence JB, Villnave C. Optimization of in situ hybridization using isotopic and non-isotopic detection methods. Biotechniques 4: 230–259, 1986.
Stefaneanu L, Kovacs K, Horvath E, Lloyd RV. In situ hybridization of pro-opio- melanocortin (POMC) gene expression in human pituitary corticotrophs and their adenomas. Virchows Arch A Pathol Anat 419: 107–113, 1991.
Guitteny AF, Fouque B, Mougin C, Teoule R, Bloch B. Histological detection of messenger RNAs with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem 36: 563–571, 1988.
Jameson JL, Klibanski A, Black PM, Zervas NT, Lindell CM, Hsu DW, Ridgway EC, Habener JF. Glycoprotein hormone genes are expressed in clinically nonfunctioning pituitary adenomas. J Clin Invest 80: 1472–1478, 1987.
Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Null cell adenomas, oncocytomas, and gonadotroph adenomas of the human pituitary: an immunocytochemical and ultrastructural analysis of 300 cases. Endocr Pathol 4: 20–27, 1993.
Klibanski A, Zervas NT, Kovacs K, Ridgway EC. Clinically silent hypersecretion of growth hormone in patients with pituitary tumors. J Neurosurg 66: 806–811, 1987.
Grossman A, Ross R, Charlesworth M, Adams CB, Wass JA, Doniach I, Besser GM. The effect of dopamine agonist therapy on large functionless pituitary tumors. Clin Endocrinol (Oxford) 22: 679–686, 1985.
Lloyd RV, Anagnostou D, Chandler WF. Dopamine receptors in immunohistochemically characterized null cell adenomas and human pituitaries. Modern Pathol 1: 51–56, 1988.
Song J, Jin L, Chandler WF, England BG, Smart JB, Landefeld TD, Lloyd RV. Gonado-tropin-releasing hormone regulates gonado-tropin β-subunit and chromogranin-B messenger ribonucleic acids in cultured chromogranin-A-positive pituitary adenomas. J Clin Endocrinol Metab 71: 622–630, 1990.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsuno, A., Teramoto, A., Takekoshi, S. et al. HGH, PRL, and ACTH gene expression in clinically nonfunctioning adenomas detected with nonisotopicIn Situ hybridization method. Endocr Pathol 6, 13–20 (1995). https://doi.org/10.1007/BF02914985
Issue Date:
DOI: https://doi.org/10.1007/BF02914985