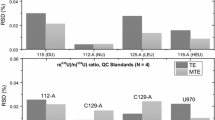
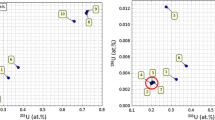
Conclusion
The published measurement data of atomic weight of uranium are shown in table 4[9]. The best results are those measured by Smith and Cowanet al. A r (U) = 238.028 79–238.028 90(3), their result error being ISD. The evaluated value of IUPAC-CAWIA is Ar(U)=238.028 9(1). In this work, the result is Ar(U) = 238.028 91(4). The combined uncertainty has been given according to 2SD of the measurement data through the calibration of a series of standard materials of uranium isotopes. It is reasonable to conclude that in this work the uncertainty which characterizes the measurement quality is better than other published data.
Similar content being viewed by others
References
IUPAC-CAWIA, Isotopic composition of the elements 1989,Pure Appl. Chem., 1991, 63: 991.
IUPAC-CAWIA, Atomic weight of the elements 1993,Pure Appl. Chem., 1994, 66: 2423.
Chang Tsinglien, Qian Qiuyu, Zhao Motianet al., The isotopic abundance of antimony,Int. J. Mass Spectrom. Ion Processes, 1993, 123: 77.
Chang Tsinglien, Qian Qiuyu, Zhao Motianel al., The absolute isotopic composition of europium,Int. J. Mass Spectrom. Ion Processes, 1994, 139: 95.
Chang Tsinglien, Qian Qiuyu, Zhao Motianet al., The absolute isotopic composition of cerium,Int. J. Mass Spectrom. Ion Processes, 1995, 142: 125.
Chang Tsinglien, Liu Yongfu, The atomic weight of cerium,Chinese Chem. Letters, 1994, 5: 357.
Cowan, G. A., Adler, H. H., The variability of the natural abundance of235U,Geochim. Cosmochim. Acta, 1976, 40: 1487.
Audi, G., Wapstra, A. H., The 1993 atomic mass evaluation,Nucl. Phys., 1993, A565: 1.
DeBievre, P., Gallet, M., Holden, N. E.et al., Isotopic abundances and atomic weights of the elements,J. Phys. Chem. Ref. Data, 1984, 13: 809.
Author information
Authors and Affiliations
About this article
Cite this article
Liu, Y., Fu, S. The isotopic abundance and atomic weight of natural uranium. Chin.Sci.Bull. 42, 814–817 (1997). https://doi.org/10.1007/BF02882489
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02882489