Summary
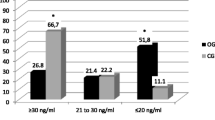
In 57 patients surgically treated for ulcer, we found low 25OHD concentrations, elevated 1,25(OH)2D concentrations, (P<0.001), a normal iPTH level, and a not significantly reduced bone mineral content (BMC) measured by single photon absorptiometry. The 25OHD concentrations were highest in patients regularly taking tablets containing vitamin D (P<0.05), whereas the 1,25 (OH)2D concentrations and the BMC values were not affected by vitamin D intake. The most severe calcium metabolic disturbances were seen in 15 Billroth I resected patients whose BMC was 89.4±12.4% of normal. Although the dietary intake of calcium and vitamin D complied with the Scandinavian recommendations and calcium absorption was in the lower part of the normal range, the general state of nutrition appears to influence the bone mass of such patients.
In the patients as a group, the 1,25(OH)2D concentrations were significantly related to BMC (r=0.42,P<0.001) and biochemical signs of bone resorption (r=0.68,P<0.001) and formation (r=0.42,P<0.001) Conversely, no relationship could be detected between serum iPTH and bone mass or bone turnover. We suggest that the high 1,25(OH)2D concentrations found after gastric resections express a compensatory process leading to an increase in calcium absorption, and that the initial event in this sequence is a trend toward low serum calcium levels. With increased demands on this regulation or lack of precursor for 1,25(OH)2D synthesis, bone mass declines through the action of 1,25(OH)2D and/or PTH.
Similar content being viewed by others
References
Aukee S, Alhava EM, Karjalainen P (1975) Bone mineral content after partial gastrectomy II. Scand J Gastroenterol 10:165–169
Tougaard L, Rickers H, Rødbro P, Hess Thaysen E, Christensen MS, Lund B, Sørensen OH (1977) Bone composition and vitamin D after Pólya gastrectomy. Acta Med Scand 202:47–50
Blichert-Toft M, Beck A, Christiansen C, Transbøl I (1979) Effects of gastric resection and vagotomy on blood and bone mineral content. World J Surg 3:99–102
Imawari M, Kozawa K, Akanuma Y, Koizumi S, Itakura H, Kosaka K (1980) Serum 25-hydrovitamin D and vitamin D-binding protein levels and mineral metabolism after partial and total gastrectomy. Gastroenterology 79:255–258
Schoen MS, Lindenbaum J, Roginsky MS, Holt PR (1978) Significance of serum level of 25-hydroxycholecalciferol in gastrointestinal disease. Am J Dig Dis 23:137–142
Nilas L, Christiansen C, Christiansen J (1985) Regulation of vitamin D and calcium metabolism after gastrectomy. Gut 26:252–257
Lilienfeld-Toal Hv, Mackes KG, Kodrat G, Ochs H, Sonnenberg A (1977) Plasma 25-hydroxyvitamin D and urinary cyclic AMP in German patients with subtotal gastrectomy (Billroth II) Am J Dig Dis 22:633–636
Garabedian M, Tanaka Y, Holick MF, DeLuca HF (1974) Response of intestinal calcium transport and bone calcium mobilization to 1,25-dihydroxyvitamin D3 in thyroparathy-roidectomized rats. Endocrinology 94:1022–1027
Gallagher JC, Riggs BL, DeLuca HF (1980) Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab 51:1359–1364
Boris A, Hurley JF, Trmal T (1979)In vivo studies in chicks and rats of bone calcium mobilization by 1α, 25-dihydroxycholecalciferol (calcitrol) and its congeners. J Nutr 109:1772–1778
Mallon JP, Matsuszewski DS, Baggiolini EG, Partridge JJ, Uskokovic MR (1981) Effect of 1α, 25-dihydroxycholecalciferol analogs on bone resorptionin vitro. J Steroid Biochem 14:599–602
Raisz LG, Trummel CL, Holick MF, DeLuca HF (1972) 1,25-dihydroxycholecalciferol: A potent stimulator of bone resorption in tissue culture. Science 175:768–769
Boris A, Hurley JF, Trmal T (1977) Relative activities of some metabolites and analogs of cholecalciferol in stimulation of tibia ash weight in chicks otherwise deprived of vitamin D. J Nutr 107:194–198
Christiansen C, Næstoft J, Hvidberg EF, Larsen N-E, Petersen B (1975) An easy procedure forin vivo estimation of protein binding and correction of elevated serum values induced by venous stasis. Clin Chim Acta 62:65–71
Shepard RM, Horst RL, Hamstra AJ, DeLuca HF (1979) Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem. J 182:55–69
Christiansen C, Baastrup PC, Lindgreen P, Transbøl I (1978) Endocrine effects of lithium: II. “Primary” hyperparathyroidism. Acta Endocrinol 88:528–534
Nilas L, Gotfredsen A, Christiansen C (1984) The relationship between local and total bone mineral content after gastric surgery. Scand J Gastroent 19:591–595
Marshall DH, Nordin BEC (1981) A comparison of radioactive calcium absorption tests with net calcium absorption Clin Sci 61:477–481
Boddy K, King PC, Hume R, Weyers E (1972) The relation of total body potassium to height, weight and age in normal adults. J Clin Pathol 25:512–517.
pødenphant J, Larsen N-E, Christiansen C (1984) Aneasy and reliable method for determination of fasting urinary hydroxyproline, an estimate of bone resorption. Clin Chim Acta 142:145–148
Statens Levnedsmiddelinstitut Ernæringsenheden (1981) Næringsstofanbefalinger og deres anvendelse Publ No 50
Pulvertaft CN (1968) Gastric resection and metabolic bone disease. Postgrad Med J 44:618–620
Clark CC (1964) Postgastrectomy bone disease. Proc Roy Soc Med 57:580–582
Kanis JA, Cundy T, Douglas D, Andrade A, Heynen G, Smith R, Russell RGG (1979) Controversies concerning the action of 1,25(OH)2D3 and 24,25(OH)2D3 in man. In: Norman AW et al. (eds) Vitamin D, basic research and its clinical application. Walter de Gruyter & Co., Berlin, New York, p 1077–1084.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nilas, L., Christiansen, C. Influence of PTH and 1,25(OH)2D on calcium homeostasis and bone mineral content after gastric surgery. Calcif Tissue Int 37, 461–466 (1985). https://doi.org/10.1007/BF02557827
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02557827