Abstract
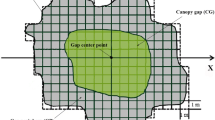
Gap characteristics and regeneration in gaps were studied in some primary evergreen broad-leaved forests of the warm temperate zone in western Japan. Total observed 161 gaps covered 15.7% of the total land area of 8.23 ha. Gap density was 19. 6 gaps ha−1 and mean gap size was 80.3 m2. Smaller gaps (<80 m2) were much more frequent than larger ones, and gaps larger than 400 m2 were rare. Gaps created by the death or the injury of single trees were 79.5%. Canopy trees died most often with broken trunks and not so often by uprooting or leaving standing-dead. Different types of gap regeneration behaviour were recognized among the major canopy tree species, though gap regeneration of the common evergreen broad-leaved tree species did not clearly depend on a species-specific gap size.Distylium racemosum, which occurred in equal importance (about 25%) in the canopy layer of each study stand, regenerates in gaps from saplings recruited before gap creation and can replace not only its own gaps but also gaps of other species. Therefore, it can be considered a typical climax species in this forest type of western Japan.Persea thunbergii, which can reproduce vegetatively, showed a similar type of gap regeneration behaviour.Castanopsis cuspidata can replace itself with relatively higher frequency by means of vegetative reproduction (stump sprouting) after gap creation.Quercus acuta andQuercus salicina did not regenerate under the current gap-disturbance regime. Though the frequency of uprooting is low, soil disturbance by uprooting seems to be important for the perpetuation of the pioneer tree species,Fagara ailanthoides, which recruits from buried seeds in the soil
Similar content being viewed by others
Abbreviations
- ha:
-
hectare (=10,000 m2)
References
Acevedo, M.F. andR. Marquis. 1978. A survey of the light gaps of the tropical rain forest at Llorona, Peninsula de Osa. Org. Trop. St.678(3): 290–303.
Asano, T. 1983. Regeneration process of beech forests. D.Sc. Thesis, Faculty of Science, Osaka City University (in Japanese).
Bormann, F.H. andG.E. Likens. 1979. Pattern and process in a forested ecosystem. Springer-Verlag, New York.
Brokaw, N.V.L. 1982. Treefalls: frequency, timing and consequences.In E.G. Leigh,et al., ed., The Ecology of a Tropical Forest: Seasonal Rhythms and Long-Term Changes, pp. 101–108. Smithonian Inst. Press, Washington.
—. 1985. Treefalls, regrowth, and community structure in tropical forests.In S.T.A. Pickett and P.S. White, ed., The Ecology of Natural Disturbance and Patch Dynamics, pp. 53–69. Academic Press, Orland.
Cottam, G. andJ.T. Curtis. 1956. The use of distance measures in phytosociological sampling. Ecology37: 451–460.
Curtis, J.T. 1959. The Vegetation of Wisconsin. Univ. of Wisconsin Press, Madison.
Denslow, J.S. 1987. Tropical rain forest gaps and tree species diversity. Annual Review of Ecology and Systematics18: 431–451.
Hartshorn, G.S. 1989. Application of gap theory to tropical forest management: natural regeneration on strip clear-cuts in the Peruvian Amazon. Ecology70: 567–569.
Hibbs, D.E. 1979. The age structure of a striped maple population. Can. J. For. Res.9: 504–508.
—,B.F. Wilson andB.C. Fisher. 1980. Habitat requirements and growth of striped maple (Acer pensylvanicum L.). Ecology61: 490–496.
Itow, S. 1972. Phytosociological studies on forest vegetation in western Kyushu, Japan. I. Natural forests ofCastanopsis cuspidata var.sieboldii. Bull. Fac. Liberal Arts, Nagasaki Univ., Nat. Sci.13: 43–50 (in Japanese with English Summary).
—. 1991. Species turnover and diversity patterns along an evergreen broad-leaved forest coenocline. Journal of Vegetation Science2: 477–484.
— andH. Nakanishi. 1987. Natural vegetation of Tsushima, Japan.In Nagasaki Prefectural Government, ed., Biogeography of the Tsushima Island, pp. 21–62. Nagasaki Prefectural Government, Nagasaki (in Japanese).
Kohyama, T. 1986. Tree size structure of stands and each species in primary warm-temperate rain forests of southern Japan. Bot. Mag. Tokyo99: 267–279.
— 1987. Stand dynamics in a primary warm-temperate rain forest analyzed by the diffusion equation. Bot. Mag. Tokyo100: 305–317.
— 1988. A function describing all-sized trunk diameter distribution in warm-temperate rain forests. Bot. Mag. Tokyo101: 207–212.
—,K. Sakamoto, T. Kobayashi andR. Watanabe. 1984. Structure of tree community of a primary lucidophyll forest in the Koyohji basin.In: M. Numata, ed., Conservation Reports of the Yakushima Wilderness Area, Kyushu, Japan. pp. 375–397. Environmental Agency of Japan, Tokyo (in Japanese with English summary).
Lawton, R.O. andF.E. Putz. 1988. Natural disturbance and gap-phase regeneration in a wind-exposed tropical cloud forest. Ecology69: 764–777.
Morita, Y. and H. Tagawa. 1981. Partial succession in gaps in aMachilus thunbelgii forest.In S. Kuroiwa, ed., Ecological Analysis of Regeneration Process and Mechanism of Forest, pp. 47–54 (in Japanese).
Mueller-Dombois, D. andH. Ellenberg. 1974. Aims and Methods of Vegetation Ecology. John Wiley & Sons, New York.
Naka, K. 1982. Community dynamics of evergreen broadleaf forests in southwestern Japan. I. Wind damaged trees and canopy gaps in an evergreen oak forest. Bot. Mag. Tokyo95: 385–399.
— andK. Yoda. 1984. Community dynamics of evergreen broadleaf forests in southwestern Japan. II. Species composition and density of seeds buried in the soil of a climax evergreen oak forest. Bot. Mag. Tokyo97: 61–79.
— andT. Yoneda. 1984. Community dynamics of evergreen broadleaf forests in southwestern Japan. III. Revegetation in gaps in an evergreen oak forest. Bot. Mag. Tokyo97: 275–286.
Nakamura, S., H. Taoda, S. Kaminaka and K. Takeshita. 1986. Natural regeneration ofCastanopsis cuspidata forest (VI) Sprouting capacities of principal trees. Trans. 39st Meet. Kyushu Br. Jpn. For. Soc.: 107–108 (in Japanese).
Nakashizuka, T. 1984. Regeneration process of climax beech (Fagus crenata Blume) forests IV. Gap formation. Jap. J. Ecol.34: 75–85.
— 1989. Role of uprooting in composition and dynamics of an old-growth forest in Japan. Ecology70: 1273–1278.
Ohkubo, T., Kaji, M. andT. Hamaya. 1988. Structure of primary Japanese beech (Fagus japonica Maxim.) forests in the Chichibu mountains, central Japan, with special reference to regeneration process. Ecol. Res.3: 101–116.
Orians, G.H. 1982. The influence of tree falls in tropical forests on tree species richness. Trop. Ecol.23: 255–279.
Putz, F.E. 1983. Treefall pits and mounds, buried seeds, and the importance of soil disturbance to pioneer tree on Barro Colorado island, Panama. Ecology64: 1069–1074.
— andK. Milton. 1982. Tree mortality rates on Barro Colorado Island.In E.G. Leigh,et al., ed., The Ecology of a Tropical Forest: Seasonal Rhythms and Long-Term Changes, pp. 95–100. Smithsonian Inst. Press, Washington.
— andN.V.L. Brokaw. 1989. Sprouting of broken trees on Barro Colorado island, Panama. Ecology70: 508–512.
Runkle, J.R. 1981. Gap regeneration in some old-growth forests of the eastern United States. Ecology62: 1041–1051.
—. 1982. Pattern of disturbance in some-old growth mesic forests of eastern North America. Ecology63: 1553–1546.
—. 1985. Disturbance regimes in temperate forests.In S.T.A. Pickett and P.S. White, ed., The Ecology of Natural Disturbance and Patch Dynamics, pp. 17–33. Academic Press, Orland.
—. 1989. Synchrony of regeneration, gaps, and latitudinal differences in tree species diversity. Ecology70: 546–547.
Spurr, S.H. andB.V. Barnes. 1980. Forest Ecology, 3 ed, John Wiley & Sons, New York.
Tagawa, H. 1977. An ecological consideration ofMachilus thunbergii forest found on Mt. Kurino, Kagoshima Prefecture. Reports from the Ebino Biological Laboratory, Kyushu University, No. 2: 31–37 (in Japanese with English summary).
Van Der Maarel, E. 1988. Vegetation dynamics: patterns in time and space. Vegetatio 77: 7–19.
Whitmore, T.C. 1989. Canopy gaps and the two major groups of forest trees. Ecology70: 536–538.
Yamamoto, S. 1989. Gap dynamics in climaxFagus crenata forests. Bot. Mag. Tokyo,102: 93–114.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamamoto, SI. Gap characteristics and gap regeneration in primary evergreen broad-leaved forests of western Japan. Bot Mag Tokyo 105, 29–45 (1992). https://doi.org/10.1007/BF02489401
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02489401