Abstract
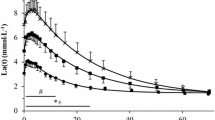
To elucidate the mechanisms of lactate formation during submaximal exercise, eight men were studied during one- (1-LE) and two-leg (2-LE) exercise (approximately 11-min cycling) using the catheterization technique and muscle biopsies (quadriceps femoris muscle). The absolute exercise intensity and thus the energy demand for the exercising limb was the same [mean 114 (SEM 7) W] during both 1-LE and 2-LE. At the end of exercise partial pressure of O2 and O2 saturation in femoral venous blood were lower and arterial adrenaline and noradrenaline were higher during 2-LE than during 1-LE. Mean arterial blood lactate concentration increased to 10.8 (SEM 0.8) (2-LE) and 5.2 (SEM 0.4) mmol · 1−1 (1-LE) after 10 min of exercise. The intramuscular metabolic response to exercise was attenuated during 1-LE [mean, lactate = 49 (SEM 9); glucose 6-P = 3.3 (SEM 0.3); nicotinamide adenine dinucleotide, reduced = 0.17 (SEM 0.02); adenosine 5′-diphosphate 2.7 (SEM 0.1) mmol · kg dry mass−1] compared to 2-LE [76 (SEM 6); 6.1 (SEM 0.7); 0.21 (SEM 0.02); 3.0 (SEM 0.1) mmol · kg dry mass−1, respectively]. To elucidate whether the lower plasma adrenaline concentration could contribute to the attenuated metabolic response, additional experiments were performed on four of the eight subjects with infusion of adrenaline during 1-LE (1-LEE). Average plasma adrenaline concentration was increased during 1-LEE and reached 2–4 times higher levels than during 2-LE. Post-exercise muscle lactate and glucose 6-P contents were higher during 1-LEE than during 1-LE and were similar to those during 2-LE. Also, leg lactate release was elevated during 1-LEE versus 1-LE. It was concluded that during submaximal dynamic exercise the intramuscular metabolic response not only depended on the muscle power output, but also on the total muscle mass engaged. Plasma adrenaline concentrations and muscle oxygenation were found to be dependent upon the working muscle mass and both may have affected the metabolic response during exercise.
Similar content being viewed by others
References
Andersen P, Saltin B (1985) Maximal perfusion of skeletal muscle in man. J Physiol (Lond) 366:233–249
Bergström J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest 68:11–13
Brooks GA (1985) Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 17:22–31
Chasiotis D, Hultman E (1985) The effect of adrenaline infusion on the regulation of glycogenolysis in human muscle during isometric contraction. Acta Physiol Scand 123:55–60
Chasiotis D, Sahlin K, Hultman E (1983) Regulation of glycogenolysis in human muscle in response to epinephrine infusion. J Appl Physiol 54:45–50
Connett RJ (1987) Glycolytic regulation during an aerobic rest-to-work transition in dog gracilis muscle. J Appl Physiol 63:2366–2374
Connett RJ, Gayeski TEJ, Honig CR (1984) Lactate accumulation in fully aerobic, working, dog gracilis muscle. Am J Physiol 246:H120-H128
Connett RJ, Honig CR, Gayeski TEJ, Brooks GA (1990) Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol 68:833–842
Davies CTM, Sargeant AJ (1974) Physiological responses to one- and two-leg exercise breathing air and 45% oxygen. J Appl Physiol 36:142–148
Gleser MA (1973) Effects of hypoxia and physical training on hemodynamic adjustments to one-legged exercise. J Appl Physiol 34:655–659
Hjemdahl P (1987) Catecholamine measurements in plasma by high-performance liquid chromatography with electrochemical detection. Methods Enzymol 142:521–534
Jansson E, Hjemdahl P, Kaijser L (1986) Epinephrine-induced changes in muscle carbohydrate metabolism during exercise in male subjects. J Appl Physiol 60:1466–1470
Jensen-Urstad M, Ahlborg G, Sahlin K (1993) High lactate and NH3 release during arm vs. leg exercise is not due to, β-adrenoceptorstimulation. J Appl Physiol 74:2860–2867
Jorfeldt L, Wahren J (1971) Leg blood flow during exercise in man. Clin Sci (Lond) 41:459–473
Katz A, Sahlin K (1987) Effect of decreased oxygen availability on NADH and lactate contents in human skeletal muscle during exercise. Acta Physiol Scand 131:119–128
Katz A, Sahlin K (1990) Role of oxygen in regulation of glycolysis and lactate production in human skeletal muscle. In: Pandolf KB, Holloszy JO (eds) Exercise and sport sciences reviews, vol 18. Williams and Wilkins, Baltimore, Md., pp 1–28
Klausen K, Secher NH, Clausen JP, Hartling O, Trap-Jensen J (1982) Central and regional circulatory adaptations to one-leg training. J Appl Physiol 52:976–983
Linnarsson D, Karlsson J, Fagraeus L, Saltin B (1974) Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol 36:399–402
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
Owles WH (1930) Alterations in the lactic acid content of the blood as a result of light exercise and associated changes in the CO2 combining power of the blood and in the alveolar CO2 pressure. J Physiol (Lond) 69:214–237
Sahlin K (1983) NADH and NADPH in human skeletal muscle at rest and during ischemia. Clin Physiol 3:477–485
Sahlin K, Katz A (1989) Hypoxemia increases the accumulation of IMP in human skeletal muscle during submaximal exercise. Acta Physiol Scand 136:199–203
Sahlin K, Katz A, Henriksson J (1987) Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem J 245:551–556
Sahlin K, Broberg S, Ren JM (1989) Formation of IMP in human skeletal muscle during incremental dynamic exercise. Acta Physiol Scand 136:193–198
Saltin B (1990) Anaerobic capacity: past, present, and prospective. In: Taylor AW, Gollnick PD, Green HJ, Ianuzzo CD, Noble EG, Métivier G, Sutton JR (eds) International series on sport sciences, vol 21. Biochemistry of exercise VII. Human Kinetics, Champaign, Ill., pp 387–412
Saltin B, Strange S (1992) Maximal oxygen uptake: “old” and “new” arguments for a cardiovascular limitation. Med Sci Sports Exerc 24:30–37
Schantz P (1986) Plasticity of human skeletal muscle: with special reference to effects of physical training on enzyme levels of the NADH shuttles and phenotypic expression of slow and fast isoforms of myofibrillar proteins. Acta Physiol Scand [Suppl] 558:6–62
Schweinsberg PD, Loo TL (1980) Simultaneous analysis of ATP, ADP, AMP and other purines in human erythrocytes by high-performance liquid chromatography. J Chromatogr 181:103–107
Spriet LL, Ren JM, Hultman E (1988) Epinephrine infusion enhances muscle glycogenolysis during prolonged electrical stimulation. J Appl Physiol 64:1439–1444
Stainsby WN, Brooks GA (1990) Control of lactate metabolism in contracting muscles and during exercise. In: Pandolf KB, Holloszy JO (eds) Exercise and sport sciences reviews. Williams and Wilkins, Baltimore, Md., pp 29–63
Svensson G, Anfält T (1982) Rapid determination of ammonia in whole blood and plasma using flow injection analysis. Clin Chim Acta 119:7–14
Vormbroch R (1984) Handling of biochemical reagents and samples. Procedures related to enzymatic analysis. In: Bergmeyer HU (ed) Methods of enzymatic analyses, vol. IV. VCH, Weinheim, pp 172–178
Wilson DF, Erecinska M, Drown C, Silver IA (1977) Effect of oxygen tension on cellular energetics. Am J Physiol 233:C135-C140
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen-Urstad, M., Svedenhag, J. & Sahlin, K. Effect of muscle mass on lactate formation during exercise in humans. Europ. J. Appl. Physiol. 69, 189–195 (1994). https://doi.org/10.1007/BF01094787
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01094787