Summary
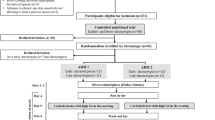
To study the islet adaptation to reduced insulin sensitivity in normal and glucose intolerant post-menopausal women, we performed a euglycaemic, hyperinsulinaemic clamp in 108 randomly selected women, aged 58–59 years. Of the 20 women with the lowest insulin sensitivity, 11 had impaired glucose tolerance (IGT) whereas 9 had normal glucose tolerance (NGT). These women together with 15 women with medium insulin sensitivity and 16 women with high insulin sensitivity and NGT were further examined with arginine stimulation at three glucose levels (fasting, 14 and >25 mmol/l). In NGT, the acute insulin response (AIR) to 5 g i. v. arginine at all three glucose levels and the slopeAIR, i. e. the glucose potentiation of insulin secretion, were markedly increased in the women with the lowest insulin sensitivity and NGT compared to those with medium or high insulin sensitivity. In contrast, in low insulin sensitivity, AIR was significantly lower in IGT than in NGT (at glucose 14 mmol/l p=0.015, and at >25 mmol/l p=0.048). The potentiation of AIR induced by low insulin sensitivity in women with NGT was reduced by 74% (AIR at 14 mmol/l glucose) and 57% (AIR at >25 mmol/l glucose), respectively, in women with IGT. Also the slopeAIR was lower in IGT than in NGT (p=0.025); the increase in slopeAIR due to low insulin sensitivity was abolished in IGT. In contrast, glucagon secretion was not different between women with IGT as opposed to NGT. We conclude that as long as there is an adequate beta-cell adaptation to low insulin sensitivity with increased insulin secretory capacity and glucose potentiation of insulin secretion, NGT persists.
Similar content being viewed by others
Abbreviations
- NIDDM:
-
Non-insulin-dependent diabetes mellitus
- AIR:
-
acute insulin response
- AGR:
-
acute glucagon response
References
Porte Jr D (1992) B cells in type 2 diabetes mellitus. Diabetes 40: 166–180
DeFronzo RA, Bonadonna RC, Ferrannini E (1992) Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15: 318–368
Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR (1992) Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340: 925–929
Lillioja S, Mott DM, Howard BV et al. (1988) Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 318: 1217–1225
Lundgren H, Bengtsson C, Blohmé G, Lapidus L, Waldenström J (1990) Fasting serum insulin concentration and early insulin response as risk determinants for developing diabetes. Diabet Med 7: 407–413
Haffner SM, Stern P, Watanabe RM, Bergman RN (1992) Relationship of insulin clearance and secretion to insulin sensitivity in non-diabetic Mexican Americans. Eur J Clin Invest 22: 147–153
O'Rahilly SP, Rudenski AS, Burnett MA, Nugent Z, Hosker JP, Darling P (1986) Beta-cell dysfunction, rather than insulin insensitivity, is the primary defect in familial type 2 diabetes. Lancet 2: 360–364
Mitrakou A, Kelley D, Mokan M et al. (1992) Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 326: 22–29
Kanatsuka A, Hideichi M, Sakurada M et al. (1988) Firstphase insulin response to glucose in nonobese or obese subjects with glucose intolerance: analysis by C-peptide secretion rate. Metabolism 37: 878–884
Sartor G, Scherstén B, Carlström S, Melander A, Norden å, Persson G (1980) Ten-year follow-up of subjects with impaired glucose tolerance: prevention of diabetes by tolbutamide and diet regulation. Diabetes 29: 41–49
Keen H, Jarrett RJ, McCartney P (1982) The ten-year follow-up of the Bedford survey (1962–1972): glucose tolerance and diabetes. Diabetologia 22: 73–78
Kadowaki T, Miyake Y, Hagura R et al. (1984) Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia 26: 44–49
Sicree RA, Zimmet PZ, King HOM, Coventry JS (1987) Plasma insulin response among Nauruans. Prediction of deterioration in glucose tolerance over 6 yr. Diabetes 36: 179–1860
Larsson H, Berglund G, Ahrén B (1995) Glucose modulation of insulin and glucagon secretion is altered in impaired glucose tolerance. J Clin Endocrinol Metab 80: 1778–1782
Cerasi E, Luft R (1967) The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol 55: 278–304
Cerasi E, Efendic S, Luft R (1973) Dose-response relation between plasma-insulin and blood-glucose levels during oral glucose loads in prediabetic and diabetic subjects. Lancet 2: 794–797
Lillioja S, Mott DM, Spraul M et al. (1993) Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329: 1988–1992
O'Meara NM, Sturis J, van Cauter E, Polonsky KS (1993) Lack of control by glucose of ultradian insulin secretory oscillations in impaired glucose tolerance and in non-insulin-dependent diabetes mellitus. J Clin Invest 92: 262–271
Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS (1995) Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest 96: 520–527
Swinburn BA, Gianchandani R, Saad MF, Lillioja S (1995) In vivo Β-cell function at the transition to early non-insulin-dependent diabetes mellitus. Metabolism 44: 757–764
Alvarsson M, Wajngot A, Efendic S, Grill V (1995) Relationship between insulin responses to D-glucose and to L-arginine in women with a history of gestational diabetes. Acta Diabetol 32: 86–91
Beard JC, Halter JB, Best JD, Pfeifer MA, Porte Jr D (1984) Dexamethasone-induced insulin resistance enhances B cell responsiveness to glucose level in normal men. Am J Physiol 247:E592-E596
Kahn SE, Beard JC, Schwartz MW et al. (1989) Increased B-cell secretory capacity as mechanism for islet adaptation to nicotinic acid-induced insulin resistance. Diabetes 38: 562–568
Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man. Measurement of insulin sensitivity and Β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214-E223
Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte Jr D (1984) Diminished B cell secretory capacity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 74: 1318–1328
Larsson H, Ahrén B, LindgÄrde F, Berglund G (1995) Fasting blood glucose in determining prevalence of diabetes in a large, homogenous population of Caucasian middle-aged women. J Intern Med 237: 537–541
World Health Organisation (1985) Diabetes mellitus: report of a WHO study group. World Health Organisation (Technical Report Series no 727), Geneva
Cerasi E (1975) Potentiation of insulin release by glucose in man. Acta Endocrinol 79: 511–534
Grill V (1981) Time and dose dependencies for priming effect of glucose on insulin secretion. Am J Physiol 240:E24–31
Halter JB, Graf RJ, Porte Jr D (1979) Potentiation of insulin secretory response by plasma glucose levels in man: Evidence that hyperglycaemia in diabetes compensates for impaired glucose potentiation. J Clin Endocrinol Metab 48: 946–954
Norusis MJ (1992) SPSS for Windows. Base system user's guide, Release 5.0. SPSS Inc., Chicago
Birkeland KI, Hanssen KF, Torjesen PA, Vaaler S (1993) Level of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab 76: 275–278
Lillioja S, Nyomba BL, Saad MF et al. (1991) Exaggerated early insulin release and insulin resistance in a diabetesprone population: a metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab 73: 866–876
Pollare T, Lithell H, Berne C (1990) Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism 39: 167–174
Campbell PJ, Gerich JE (1990) Impact of obesity on insulin action in volunteers with normal glucose tolerance: demonstration of a threshold for the adverse effect of obesity. J Clin Endocrinol Metab 70: 1114–1118
Reaven GM, Hollenbeck CB, Chen YDI (1989) Relationship between glucose tolerance, insulin secretion, and insulin action in non-obese individuals with varying degrees of glucose tolerance. Diabetologia 32: 52–55
Haffner SM, KarhapÄÄ P, MykkÄnen L, Laakso M (1994) Insulin resistance, body fat distribution and sex hormones in men. Diabetes 43: 212–219
Groop LC, Widén E, Ferrannini E (1993) Insulin resistance and insulin deficiency in the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus: errors of metabolism or of methods? Diabetologia 36: 1326–1331
Sbraccia P, D'Adamo M, Leonetti F et al. (1996) Chronic primary hyperinsulinaemia is associated with altered insulin receptor mRNA splicing in muscle of patients with insulinoma. Diabetologia 39: 220–226
Faber OK, Christensen K, Kehlet H, Madsbad S, Binder C (1981) Decreased insulin removal contributes to hyperinsulinaemia in obesity. J Clin Endocrinol Metab 53: 618–621
Kautzky-Willer A, Pacini G, Ludvik B, Schernthaner G, Prager R (1992) B-cell hypersecretion and not reduced hepatic insulin extraction is the main cause of hyperinsulinaemia in obese nondiabetic subjects. Metabolism 41: 1304–1312
Haffner SM, Bowsher RR, MykkÄnen L et al. (1994) Proinsulin and specific insulin concentration in high- and low-risk populations for NIDDM. Diabetes 43: 1490–1493
Kahn SE, Leonetto DL, Prigeon RL, Boyko EJ, Bergstrom RW, Fujimoto WY (1995) Proinsulin as a marker for the development of NIDDM in Japenese-American men. Diabetes 44: 173–179
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Larsson, H., Ahrén, B. Failure to adequately adapt reduced insulin sensitivity with increased insulin secretion in women with impaired glucose tolerance. Diabetologia 39, 1099–1107 (1996). https://doi.org/10.1007/BF00400660
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00400660