Summary
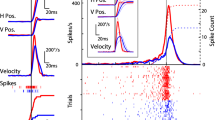
Anatomical and electrophysiological data have shown that, in the guinea pig as well as in the cat, the nucleus prepositus hypoglossi gives rise to a disynaptic ascending projection to the superior colliculus via the peri-parabigeminal area in the mesencephalon. The functional role of this indirect pathway in the generation of eye movements has been studied by pharmacologically interfering at the mesencephalic level and by examining the induced effects on two differently elicited saccades in the alert guinea pig. A small iontophoretic injection of kainic acid induces a transient increase of the spontaneous saccadic activity in the contraversive direction leading to a pseudo-nystagmus. Both the amplitude and the mean velocity of the contraversive saccades are greater than before the injection. A similar enhancement also affects the parameters of the contraversive saccades induced by trunk rotations. These results suggest that peri-parabigeminal neurones exert an excitatory influence on their target cells in the ipsilateral superior colliculus which is responsible for contraversive rapid eye movements.
Similar content being viewed by others
References
Barmack NH, Nastos MA, Pettorossi VE (1981) The horizontal and vertical cervico-ocular reflexes of the rabbit. Brain Res 224:261–278
Beninato M, Spencer RF (1986) A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acethyltransferase immunochemistry. J Comp Neurol 253:525–538
Boussaoud D, Joseph JP (1985) role of the cat substantia nigra pars reticulata in eye and head movements. II. Effects of local pharmacological injections. Exp Brain Res 57:297–304
Edwards SB, Ginsburgh CL, Henkel CK, Stein BE (1979) Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol 184:309–330
Flandrin JM, Jeannerod M (1981) Effects of unilateral superior colliculus ablation on oculomotor and vestibulo-ocular responses in the cat. Exp Brain Res 42:73–80
Grantyn A, Grantyn R (1982) Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tectobulbo-spinal tract. Exp Brain Res 46:243–256
Graybiel AM (1977) Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol 175:37–78
Gresty MA (1976) A reexamination of “neck reflex” eye movements in the rabbit. Acta Otolaryngol 81:386–394
Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D (1989) Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol 287:495–514
Hardy O, Mirenowicz J (in press) Role of the lateral mesencephalic reticular formation in the control of head movements. In: Berthoz A, Graf WM, Vidal PP (eds) The head-neck sensory motor system. Oxford University Press, New York
Hardy O, Schalchli L (1989) Head velocity sensitivity of some lateral posterior mesencephalic neurones in alert head-fixed guinea pig. Abstr Eur J Neurosci Suppl 2:36
Henkel CK (1981) Afferent sources of a lateral midbrain tegmental zone associated with the pinnae in the cat as mapped by retrograde transport of horseradish peroxidase. J Comp Neurol 203:213–226
Hikosaka O, Igusa Y (1980) Axonal projection of prepositus hypoglossi and reticular neurons in the brainstem of the cat. Exp Brain Res 39:441–451
Huerta MF, Harting JK (1984) The mammalian superior colliculus: Studies of its morphology and connections. In: Vanegas H (eds) Comparative neurology of the optic tectum. Plenum Press, New York, pp 687–773
McCrea RA, Baker R (1985) Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol 237:377–407
Moschovakis AK, Karabelas AB, Highstein SM (1988) Structure-function relationship in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol 60:263–302
Ohki Y, Shimazu H, Suzuki I (1988) Excitatory input to burst neurons from labyrinth and its mediating pathway in the cat: location and functional characteristics of burster-driving neurons. Exp Brain Res 72:457–472
Sakamoto M, Hikosaka O (1989) Eye movements induced by microinjection of GABA agonist in the rat substantia nigra pars reticulata. Neurosci Res 6:216–233
Satoh K, Fibiger HC (1986) Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol 253:277–302
Schaefer KP, Meyer DL (1974) Compensation of vestibular lesions. In: Kornhuber HH (eds) Handbook of sensory physiology. Springer, Berlin, pp 463–490
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hardy, O., Mirenowicz, J. Transient increase of contraversive saccade parameters following kainic acid injection in the periparabigeminal area of guinea pig. Exp Brain Res 85, 616–620 (1991). https://doi.org/10.1007/BF00231746
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231746