Summary
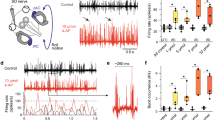
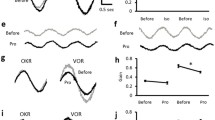
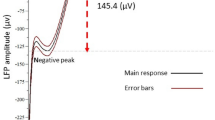
It has been proposed that a common velocity-storage mechanism is responsible for the prolongation of vestibular nystagmus beyond the duration of the change in firing frequency of primary vestibular fibers in response to a step in velocity, and for the production of optokinetic afternystagmus (OKAN). In a previous study, bilateral injection of the aselective cholinergic agonist carbachol in the flocculus shortened the duration of buildup of optokinetic nystagmus (OKN) and the duration of OKAN, suggesting floccular involvement in velocity storage (Tan et al. 1992). In extension to that study of OKN, the present study assesses the effects of floccular carbachol on vestibular nystagmus in response to velocity steps. Our results show that injection of carbachol shortens the duration of vestibular nystagmus from about 13 to about 8 s; a finding which supports a common velocity-storage mechanism for optokinetic and vestibular signals. We propose that the indistinguishable effects of carbachol on OKAN and vestibular nystagmus are due to modification of the transmission of an oculomotor corollary signal, which has been identified electrophysiologically in the flocculus.
Similar content being viewed by others
References
Barmack NH, Pettorossi VE (1985) Effects of unilateral lesions of the flocculus on optokinetic and vestibuloocular reflexes of the rabbit. J Neurophysiol 53: 481–496
Blanks RHI, Estes MS, Markham CH (1975) Physiologic characteristics of vestibular first order canal neurons in the cat. II. Response to constant angular acceleration. J Neurophysiol 38: 1250–1268
Collewjjn H (1969) Optokinetic eye movements in the rabbit: input-output relations. Vision Res 9: 117–132
Collewijn H, Kleinschmidt HJ (1975) Vestibulo-ocular and optokinetic reactions in the rabbit: changes during 24 hours of normal and abnormal interaction. In: Lennerstrand G, Bach-y-Rita P (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon Press, Oxford New York, pp 477–483
Collewijn H, Winterson BJ, Van der Steen J (1980) Post-rotatory nystagmus and optokinetic after-nystagmus in the rabbit: linear rather than exponential decay. Exp Brain Res 40: 330–338
Fernández C and Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34: 661–675
Ito M (1972) Neural design of the cerebellar motor control system. Brain Res 40: 81–84
Ito M (1977) Neuronal events in the cerebellar flocculus associated with an adaptive modification of the vestibulo-ocular reflex of the rabbit. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons, Elsevier, Amsterdam, New York, pp 391–398
Ito M, Jastreboff PJ, Miyashita Y (1982) Specific effects of unilateral lesions in the flocculus upon eye movements in albino rabbits. Exp Brain Res 45: 233–242
Jung R (1948) Die Registrierung des postrotatorischen und optokinetischen Nystagmus und die optisch-vestibuläre Integration beim Menschen. Acta Otolaryngol (Stockh) 36: 199–202
Leonard CS (1986) Signal characteristics of cerebellar Purkinje cells in the rabbit flocculus during compensatory eye movements. PhD Dissertation, New York University, New York
Leonard CS, Simpson JI (1984) Purkinje cell activity in the flocculus of the alert rabbit during natural visual and vestibular stimulation. Soc Neurosci Abstr 10: 538
Leonard CS, Simpson JI (1985) Purkinje cell activity in the flocculus of the alert rabbit during natural visual and vestibular stimulation, part 2. Soc Neurosci Abstr 11: 1033
Lisberger SG, Fuchs AF (1978) Role of the primate flocculus during rapid behavioural modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive rotation. J Neurophysiol 41: 733–777
Miles FA, Fuller JH (1975) Visual tracking and the primate flocculus. Science 189: 1000–1002
Miles FA, Fuller JH, Braitman DJ, Dow BM (1980) Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol 43: 1437–1476
Mowrer OH (1937) The influence of vision during bodily rotation upon the duration of post-rotational vestibular nystagmus. Acta Otolaryngol (Stockh) 25: 351–364
Nagao S (1983) Effects of vestibulocerebellar lesions upon dynamic characteristics and adaptation of vestibulo-ocular and optokinetic responses in pigmented rabbits. Exp Brain Res 53: 36–46
Raphan T, Cohen B (1985) Velocity storage and the ocular response to multidimensional vestibular stimuli. In: Berthoz A, Melvill-Jones G (eds) Adaptive mechanisms in gaze control. Elsevier, Amsterdam New York, pp 123–143
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145
Robinson DA (1976) Adaptive gain control of the vestibulo-ocular reflex by the cerebellum. J Neurophysiol 39: 954–969
Robinson DA (1977) Vestibular and optokinetic symbiosis: an example of explaining by modelling. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons. Elsevier, Amsterdam New York, pp 49–58
Schmid R, Jeannerod M (1985) Vestibular habituation: an adaptive process? In: Berthoz A, Melvill-Jones G (eds) Adaptive mechanisms in gaze control. Elsevier, Amsterdam New York, pp 113–122
Skavenski AA, Blair SM, Westheimer G (1981) The effect of habituating vestibular and optokinetic nystagmus on each other. J Neurosci 1: 351–357
Steinhausen W (1933) Über die Beobachtung der Cupula in den Bogengangsampullen des Labyrinths des lebenden Hechts. Pflügers Arch 232: 500–512
Tan HS, Collewijn H (1991) Cholinergic modulation of optokinetic and vestibulo-ocular responses: a study with microinjections in the flocculus of the rabbit. Exp Brain Res 85: 475–481
Tan HS, Collewijn H, Van der Steen J (1992) Optokinetic nystagmus in the rabbit and its modulation by bilateral microinjection of carbachol in the cerebellar flocculus. Exp Brain Res 90: 456–468
Ter Braak JWG (1936) Untersuchungen über optokinetischen Nystagmus. Arch Neerl Physiol 21: 309–376
Van Neerven J, Pompeiano O, Collewijn H (1989) Depression of the vestibulo-ocular and optokinetic response by intrafloccular microinjection of GABA-A and GABA-B agonists in the rabbit. Arch Ital Biol 127: 243–263
Waespe W, Kenn V (1981) Visual-vestibular interaction in the flocculus of the alert monkey. II. Purkinje cell activity. Exp Brain Res 43: 349–360
Waespe W, Cohen B, Raphan T (1983) Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res 50: 9–33
Zee DS, Yamazaki A, Butler PH, Güçer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46: 878–899
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tan, H.S., Collewijn, H. & Van der Steen, J. Shortening of vestibular nystagmus in response to velocity steps by microinjection of carbachol in the rabbit's cerebellar flocculus. Exp Brain Res 92, 385–390 (1993). https://doi.org/10.1007/BF00229026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229026