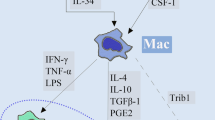
Abstract
Human tumor tissues can often be anatomically classified into areas of cancer nest, invading edge, and peritumoral stroma, each with distinct compositions and functional properties. Macrophages (Mφ) constitute a major component of the leukocyte infiltrate in tumors. These cells are derived from circulating monocytes, and in response to environmental signals, they exhibit distinct phenotypes with diverse functions. Soluble factors derived from cancer cells can alter the normal developmental process of Mφ that is intended to trigger transient early activation of monocytes in the peritumoral region, which in turn induces formation of suppressive Mφ in cancer nests. The activated monocytes in the peritumoral region attenuated the T-cell response by expressing B7-H1, and were superior to the suppressive tumor Mφ in inducing Th17 expansion, and thus repurpose the inflammatory response away from anti-tumor immunity (the sword) and towards tissue remodeling and proangiogenic pathways (a plowshare). In contrast, the suppressive Mφ can induce the production of Tregs in cancer nest. Accordingly, angiogenesis was most active at the invading edge, which was situated close to the peritumoral stroma with activated Mφ and the density of these activated monocytes is selectively associated with vascular invasion and metastasis in patients with hepatocellular carcinoma. These data reveal an intriguing mechanism in which human Th17 cells are generated and regulated by a fine-tuned collaborative action between different types of immune cells in distinct tumor microenvironments. These results give important new insights into the distinct role of macrophages in human tumor progression which would be helpful for the rational design of novel immune-based anticancer therapies.

Similar content being viewed by others
References
Mueller MM, Fusenig NE (2004) Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4:839–849
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
van Zijl F, Mair M, Csiszar A et al (2009) Hepatic tumor-stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene 28:4022–4033
Tlsty TD, Coussens LM (2006) Tumor stroma and regulation of cancer development. Annu Rev Pathol 1:119–150
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5:263–274
Karin M, Lawrence T, Nizet V (2006) Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124:823–835
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–612
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Biswas SK, Mantovani A (2011) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896
Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8:618–631
Budhu A, Forgues M, Ye QH et al (2006) Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 10:99–111
Vakkila J, Lotze MT (2004) Inflammation and necrosis promote tumour growth. Nat Rev Immunol 4:641–648
Qian BZ, Pollard JW (2011) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Gao B, Jeong WI, Tian Z (2008) Liver: an organ with predominant innate immunity. Hepatology 47:729–736
Liu H, Pan Z, Li A et al (2008) Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol 5:373–378
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917
Xu J, Ding T, He Q et al (2012) In situ molecular signature predict early recurrence in Hepatitis B virus-related hepatocellular carcinoma. J Hepatol, in press
Shi C, Pamer EG (2011) Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774
Taylor PR, Martinez-Pomares L, Stacey M et al (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944
Lawrence T, Natoli G (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11:750–761
Kuang DM, Wu Y, Chen N et al (2007) Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 110:587–595
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Krausgruber T, Blazek K, Smallie T et al (2011) IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 12:231–238
He M, Xu Z, Ding T, Kuang DM, Zheng L (2009) MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol Immunol 6:343–352
Cheng J, Huo DH, Kuang DM et al (2007) Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Cancer Res 67:5141–5147
Yang M, Chen J, Su F et al (2011) Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 10:117
Lewis CE, De Palma M, Naldini L (2007) Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res 67:8429–8432
Qian BZ, Li J, Zhang H et al (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225
Fu J, Xu D, Liu Z et al (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132:2328–2339
Gao Q, Qiu SJ, Fan J et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Zhou J, Ding T, Pan W et al (2009) Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 125:1640–1648
Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P (2009) Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 9:445–452
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774
Godin-Ethier J, Pelletier S, Hanafi LA et al (2009) Human activated T lymphocytes modulate IDO expression in tumors through Th1/Th2 balance. J Immunol 183:7752–7760
Zhao Q, Kuang DM, Wu Y et al (2012) Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol 188:1117–1124
Kuang DM, Peng C, Zhao Q et al (2010) Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 51:154–164
Ding T, Xu J, Wang F et al (2009) High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol 40:381–389
Kuang DM, Peng C, Zhao Q et al (2010) Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol 185:1544–1549
Kuang DM, Zhao Q, Peng C et al (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 206:1327–1337
Kuang DM, Zhao Q, Wu Y et al (2011) Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol 54:948–955
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8:467–477
Hou J, Tian L, Wei Y (2004) Cancer immunotherapy of targeting angiogenesis. Cell Mol Immunol 1:161–166
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370
Lewis CE, Hughes R (2007) Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res 9:209
Lewis C, Murdoch C (2005) Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol 167:627–635
Xu S, Cao X (2010) Interleukin-17 and its expanding biological functions. Cell Mol Immunol 7:164–174
Zou W, Restifo NP (2010) T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 10:248–256
Zhang JP, Yan J, Xu J et al (2009) Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 50:980–989
Su X, Ye J, Hsueh EC et al (2010) Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol 184:1630–1641
Yen HR, Harris TJ, Wada S et al (2009) Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol 183:7161–7168
Li J, Huang ZF, Xiong G et al (2011) Distribution, characterization, and induction of CD8 regulatory T cells and IL-17-producing CD8 T cells in nasopharyngeal carcinoma. J Transl Med 9:189
Wang L, Yi T, Kortylewski M et al (2009) IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 206:1457–1464
Kryczek I, Wei S, Zou L et al (2007) Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 178:6730–6733
Muranski P, Boni A, Antony PA et al (2008) Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112:362–373
Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6:173–182
Wu Y, Zhao Q, Peng C et al (2011) Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol 225:438–447
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Kessenbrock K, Plaks V, Werb Z (2011) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67
Shi GM, Ke AW, Zhou J et al (2010) CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology 52:183–196
Zhao Q, Xiao X, Wu Y et al (2011) Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol 41:2314–2322
Acknowledgments
This work was supported by project grants from the National Natural Science Foundation of China (91029737), and the “973” Program (2010CB529904 and 2011CB811305).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Zheng, L. Dynamic Education of Macrophages in Different Areas of Human Tumors. Cancer Microenvironment 5, 195–201 (2012). https://doi.org/10.1007/s12307-012-0113-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-012-0113-z