Abstract
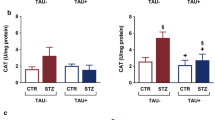
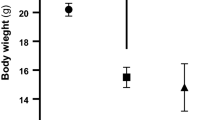
The aim of the current study was to evaluate the role of γ-amino butyric acid (GABA) in insulin disturbance and hyperglycemia associated with brain oxidative damage in streptozotocin-treated rats. Streptozotocin (STZ) was administered to male albino rats as a single intraperitoneal dose (60 mg/kg body weight). GABA (200 mg/Kg body weight/day) was administered daily via gavages during 3 weeks to STZ-treated-rats. Male albino rats Sprague–Dawley (10 ± 2 weeks old; 120 ± 10 g body weight) were divided into 4 groups of 6 rats and treated in parallel. (1) Control group: received distilled water, (2) GABA group: received GABA, (3) STZ group: STZ-treated rats received distilled water, (4) STZ + GABA group: STZ-treated rats received GABA. Rats were sacrificed after a fasting period of 12 h next last dose of GABA. The results obtained showed that STZ-treatment produced hyperglycemia and insulin deficiency (similar to type1 Diabetes). These changes were associated with oxidative damage in brain tissue and notified by significant decreases of superoxide dismutase and catalase activities in parallel to significant increases of malondialdehyde and advanced oxidation protein products levels. The histopathology reports also revealed that STZ-treatment produced degeneration of pancreatic cells. The administration of GABA to STZ-treated rats preserved pancreatic tissue with improved insulin secretion, improved glucose level and minimized oxidative stress in brain tissues. It could be concluded that GABA might protect the brain from oxidative stress and preserve pancreas tissues with adjusting glucose and insulin levels in Diabetic rats and might decrease the risk of neurodegenerative disease in diabetes.


Similar content being viewed by others
References
Chintan AP, Nimish LP, Nayana B, Bhavna M, Mahendra G, Hardik T. Cardiovascular complication of diabetes mellitus. J Appl Pharm Sci. 2011;4:1–6.
Haskins K, Bradley B, Powers K. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54.
Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–9.
Wang JY, Zhu C, Qian TW, Guo H, Wang DD, Zhang F, et al. Extracts of black bean peel and pomegranate peel ameliorate oxidative stress-induced hyperglycemia in mice. Exp Ther Med. 2015;9(1):43–8.
Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabeticencephalopathy. Brain. 2008;131(12):3311–34.
Zhou Y, Luo Y, Dai J. Axonal and dendritic changes are associated with diabetic encephalopathy in rats: an important risk factor for Alzheimer’s disease. J Alzheimers Dis. 2013;34:937–47.
Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur. J Pharmacol. 2002;441:1–14.
Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, et al. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology. 2009;10(4):377–413.
Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32(9):1371–82.
Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–93.
Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–64.
Migliore L, Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84.
Mohamadin AM, Sheikh B. Abd El-Aal AA, Elberry AA, Al-Abbasi FA. Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions. Pest Biochem Physiol. 2010;98:128–34.
Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants: a review. J Biochem Mol Toxic. 2003;17:24–38.
Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47.
Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221.
Billinton A, Ige AO, Bolam JP, White JH, Marshall FH, Emson PC. Advances in the molecular understanding of GABAB receptors. Trends Neurosci. 2001;24:277–82.
Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–40.
Krnjevic K. How does a little acronym become a big transmitter? Biochem Pharmacol. 2004;68:1549–55.
Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43.
Soi-ampornkul R, Junnu S, Kanyok S, Liammongkolkul S, Katanyoo W, Umpornsirirat S. Antioxidative and neuroprotective activities of the pre-germinated brown rice extract. Food Nutr Sci. 2012;3(1):135–40. doi:10.4236/fns.2012.31020.
Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–27.
Minuk GY. Gamma-aminobutyric acid and the liver. Dig Dis. 1993;11(1):45–54.
Erdo SL, Wolf JR. γ-Aminobutyric acid outside the mammalian brain. J Neurochem. 1990;54:363–72.
Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59:1694–701.
Bergersen LH, Storm-Mathisen J, Gundersen V. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc. 2008;3:144–52.
Erejuwa OO, Sulaiman SA, Wahab MSA, Sirajudeen KNS, Salleh MSM, Gurtu S. Glibenclamide or metformin combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int J Biol Sci. 2011;72:244–52.
Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Allah A, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22(2):60–4.
Nakagawa T, Yokozawa T, Kim HJ, Shibahara N. Protective effect of gamma aminobutyric acid in rats with streptozotocin-induced diabetes. J Nutr Sci Vitaminol. 2005;51:278–82.
Mikesh LM, Bruns DE. Stabilization of glucose in blood specimens: mechanism of delay in fluoride inhibition of glycolysis. Clin Chem. 2008;54(5):930–2.
Trinder P. Enzymatic colorimetric determination of glucose. Ann Clin Biochem. 1969;6(2):24–7.
Clark PMS, Hales CN. How to measure plasma insulin. Diabetes Metab Rev. 1994;10:79–90.
Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–6.
Witko-Sarsat V, Friendlander M, Capelliere-Blandin C, Nguyen-KhoaT Zing J, Jungers P, et al. Advanced oxidation protein products as a noval marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13.
Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–54.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94.
Bancroft JD, Gamble M. Theory and practice of histological techniques. In: 5th ed. Edinburgh. New York, London, Philadelphia, Churchill Livingstone Pub; 2002. pp. 172–175 and 593–620.
Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med. 2012;237:481–90.
Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48(4):927–32.
Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38.
Young TA, Cunningham CC, Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys. 2002;405(1):65–72.
Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10(14):1611–26.
Davi G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–68.
Yoshida S, Hashimoto T, Kihara M, Imai N, Yasuzaki H, Nomura K, et al. Urinary oxidative stress markers closely reflect the efficacy of Candesartan treatment for diabetic nephropathy. Nephron Exp Nephrol. 2008;111:20–30.
Sadi G, Yilmaz O, Güray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol Cell Biochem. 2008;309:109–16.
Sadi G, Guray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: effect of antioxidants. Mol Cell Biochem. 2009;327:127–34.
Matsunami T, Sato Y, Sato T, Ariga S, Shimomura T, Yukawa M. Oxidative stress and gene expression of antioxidant enzymes in the streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2010;3(2):177–88.
Yoshikuni Y. FDA GRAS Notice for gamma-amino butyric acid (GABA). Kyoto: Pharma Foods International Co. Ltd; 2008.
Palak P, Dipa I. GABA as potential target in the treatment of Type-1 diabetes mellitus. IAJPR. 2013; 3(3):2636–2642. www.scopemed.org/?mno=155108. Accessed 11 July 2016.
Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci. 2011;108(28):11692–7.
Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes. 2014;63(12):4197–205. doi:10.2337/db14-0153 Epub 2014 Jul 9.
Bansal P, Wang S, Liu S, Xiang YY, Lu WY, Wang Q. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 β-cells. PLoS One. 2011;6(10):26225.
Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58.
Hou CW. Pu-Erh tea and GABA attenuates oxidative stress in kainic acid-induced status epilepticus. J Biomed Sci. 2011;18:75.
Deng Y, Wang W, Yu P, Xi Z, Xu L, Li X, et al. Comparison of taurine, GABA, Glu, and Asp as scavengers of malondialdehyde in vitro and in vivo. Nanoscale Res Lett. 2013;8:190.
Sasaki S, Yokozawa T, Cho EJ, Oowada S, Kim M. Protective role of gamma-aminobutyric acid against chronic renal failure in rats. J Pharm Pharmacol. 2006;11:1515–25.
Liu B, Barbosa-Sampaio H, Jones PM, Persaud SJ, Muller DS. The CaMK4/CREB/IRS-2 cascade stimulates proliferation and inhibits apoptosis of β-cells. PLoS One. 2012;7(9):e45711. doi:10.1371/journal.pone.0045711.
Prud’homme GJ, Glinka Y, Udovyk O, Hasilo C, Paraskevas S, Wang Q. GABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activity. Biochem Biophys Res Commun. 2014;452(3):649–54.
Tian J, Dang HN, Yong J, Chui WS, Dizon MP, Yaw CK, et al. oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS One. 2011;6(9):25338.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Eltahawy, N.A., Saada, H.N. & Hammad, A.S. Gamma Amino Butyric Acid Attenuates Brain Oxidative Damage Associated with Insulin Alteration in Streptozotocin-Treated Rats. Ind J Clin Biochem 32, 207–213 (2017). https://doi.org/10.1007/s12291-016-0597-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0597-2