Abstract
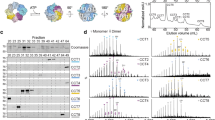
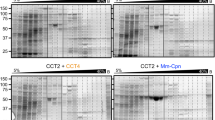
Archaeal and eukaryotic cytosols contain group II chaperonins, which have a double-barrel structure and fold proteins inside a cavity in an ATP-dependent manner. The most complex of the chaperonins, the eukaryotic TCP-1 ring complex (TRiC), has eight different subunits, chaperone containing TCP-1 (CCT1–8), that are arranged so that there is one of each subunit per ring. Aspects of the structure and function of the bovine and yeast TRiC have been characterized, but studies of human TRiC have been limited. We have isolated and purified endogenous human TRiC from HeLa suspension cells. This purified human TRiC contained all eight CCT subunits organized into double-barrel rings, consistent with what has been found for bovine and yeast TRiC. The purified human TRiC is active as demonstrated by the luciferase refolding assay. As a more stringent test, the ability of human TRiC to suppress the aggregation of human γD-crystallin was examined. In addition to suppressing off-pathway aggregation, TRiC was able to assist the refolding of the crystallin molecules, an activity not found with the lens chaperone, α-crystallin. Additionally, we show that human TRiC from HeLa cell lysate is associated with the heat shock protein 70 and heat shock protein 90 chaperones. Purification of human endogenous TRiC from HeLa cells will enable further characterization of this key chaperonin, required for the reproduction of all human cells.







Similar content being viewed by others
References
Acosta-Sampson L, King J (2010) Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone. J Mol Biol 401(1):134–152
Almeida MB, do Nascimento JL, Herculano AM, Crespo-López ME (2011) Molecular chaperones: toward new therapeutic tools. Biomed Pharmacother 65(4):239–243
Bigotti MG, Clarke AR (2008) Chaperonins: the hunt for the group II mechanism. Arch Biochem Biophys 474(2):331–339
Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB (1994) The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature 371(6498):578–586
Cong Y, Baker ML, Jakana J, Woolford D, Miller EJ, Reissmann S, Kumar RN, Redding-Johanson AM, Batth TS, Mukhopadhyay A, Ludtke SJ, Frydman J, Chiu W (2010) 4.0-A resolution cryo-EM structure of the mammalian chaperonin TRiC/CCT reveals its unique subunit arrangement. Proc Natl Acad Sci USA 107(11):4967–4972
Dekker C, Roe SM, McCormack EA, Beuron F, Pearl LH, Willison KR (2011) The crystal structure of yeast CCT reveals intrinsic asymmetry of eukaryotic cytosolic chaperonins. EMBO J 30(15):3078–3090
Douglas NR, Reissmann S, Zhang J, Chen B, Jakana J, Kumar R, Chiu W, Frydman J (2011) Dual action of ATP hydrolysis couples lid closure to substrate release into the group II chaperonin chamber. Cell 144(2):240–252
Evans P, Slingsby C, Wallace BA (2008) Association of partially folded lens betaB2-crystallins with the alpha-crystallin molecular chaperone. Biochem J 409(3):691–699
Feldman DE, Spiess C, Howard DE, Frydman J (2003) Tumorigenic mutations in VHL disrupt folding in vivo by interfering with chaperonin binding. Mol Cell 12(5):1213–1224
Ferreyra RG, Frydman J (2000) Purification of the cytosolic chaperonin TRiC from bovine testis. Methods Mol Biol 140:153–160
Flaugh SL, Kosinski-Collins MS, King J (2005) Contributions of hydrophobic domain interface interactions to the folding and stability of human gammaD-crystallin. Protein Sci 14(3):569–581
Fountoulakis M, Tsangaris G, Oh J-e, Maris A, Lubec G (2004) Protein profile of the HeLa cell line. J Chromatogr A 1038(1–2):247–265
Frydman J (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70:603–647
Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall J, Tempst P, Hartl F (1992) Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J 11(13):4767–4778
Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU (1994) Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370(6485):111–117
Gao Y, Thomas J, Chow R, Lee G, Cowan N (1992) A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell 69(6):1043–1050
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332
Hoehenwarter W, Tang Y, Ackermann R, Pleissner K-P, Schmid M, Stein R, Zimny-Arndt U, Kumar NM, Jungblut PR (2008) Identification of proteins that modify cataract of mouse eye lens. Proteomics 8(23–24):5011–5024
Horwich AL, Fenton WA, Chapman E, Farr GW (2007) Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol 23:115–145
Horwitz J (1992) Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA 89(21):10449–10453
Hynes GM, Willison KR (2000) Individual subunits of the eukaryotic cytosolic chaperonin mediate interactions with binding sites located on subdomains of beta-actin. J Biol Chem 275(25):18985–18994
Kabir MA, Uddin W, Narayanan A, Reddy PK, Jairajpuri MA, Sherman F, Ahmad Z (2011) Functional subunits of eukaryotic chaperonin CCT/TRiC in protein folding. J Amino Acids 2011:843206
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111
Kim S, Willison K, Horwich A (1994) Cytosolic chaperonin subunits have a conserved ATPase domain but diverged polypeptide-binding domains. Trends Biochem Sci 19(12):543–548
Kitamura A, Kubota H, Pack C-G, Matsumoto G, Hirayama S, Takahashi Y, Kimura H, Kinjo M, Morimoto RI, Nagata K (2006) Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat Cell Biol 8(10):1163–1170
Knee KM, Goulet DR, Zhang J, Chen B, Chiu W, King JA (2011) The group II chaperonin Mm-Cpn binds and refolds human γD crystallin. Protein Sci 20(1):30–41
Kosinski-Collins MS, King J (2003) In vitro unfolding, refolding, and polymerization of human gammaD crystallin, a protein involved in cataract formation. Protein Sci 12(3):480–490
Kubota H, Yokota S, Yanagi H, Yura T (1999) Structures and co-regulated expression of the genes encoding mouse cytosolic chaperonin CCT subunits. Eur J Biochem 262(2):492–500
Leitner A, Joachimiak LA, Bracher A, Mönkemeyer L, Walzthoeni T, Chen B, Pechmann S, Holmes S, Cong Y, Ma B, Ludtke S, Chiu W, Hartl FU, Aebersold R, Frydman J (2012) The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure 20(5):814–825
Lewis V, Hynes G, Dong Z, Saibil H, Willison K (1992) T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature 358(6383):249–252
Llorca O, Martín-Benito J, Gómez-Puertas P, Ritco-Vonsovici M, Willison KR, Carrascosa JL, Valpuesta JM (2001) Analysis of the interaction between the eukaryotic chaperonin CCT and its substrates actin and tubulin. J Struct Biol 135(2):205–218
Machida K, Masutani M, Kobayashi T, Mikami S, Nishino Y, Miyazawa A, Imataka H (2012) Reconstitution of the human chaperonin CCT by co-expression of the eight distinct subunits in mammalian cells. Protein Expr Purif 82(1):61–69
Mitchell P, Cumming RG, Attebo K, Panchapakesan J (1997) Prevalence of cataract in Australia: the Blue Mountains eye study. Ophthalmology 104(4):581–588
Moreau KL, King JA (2012a) Cataract-causing defect of a mutant γ-crystallin proceeds through an aggregation pathway which bypasses recognition by the α-crystallin chaperone. PLoS One 7(5):e37256
Moreau KL, King JA (2012b) Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med 18(5):273–282
Muñoz IG, Yébenes H, Zhou M, Mesa P, Serna M, Park AY, Bragado-Nilsson E, Beloso A, de Cárcer G, Malumbres M, Robinson CV, Valpuesta JM, Montoya G (2011) Crystal structure of the open conformation of the mammalian chaperonin CCT in complex with tubulin. Nat Struct Mol Biol 18(1):14–19
Neirynck K, Waterschoot D, Vandekerckhove J, Ampe C, Rommelaere H (2006) Actin interacts with CCT via discrete binding sites: a binding transition-release model for CCT-mediated actin folding. J Mol Biol 355(1):124–138
Nimmesgern E, Hartl FU (1993) ATP-dependent protein refolding activity in reticulocyte lysate. Evidence for the participation of different chaperone components. FEBS Lett 331(1–2):25–30
Norcum MT (1996) Novel isolation method and structural stability of a eukaryotic chaperonin: the TCP-1 ring complex from rabbit reticulocytes. Protein Sci 5(7):1366–1375
Pappenberger G, McCormack EA, Willison KR (2006) Quantitative actin folding reactions using yeast CCT purified via an internal tag in the CCT3/gamma subunit. J Mol Biol 360(2):484–496
Pereira JH, Ralston CY, Douglas NR, Kumar R, Lopez T, McAndrew RP, Knee KM, King JA, Frydman J, Adams PD (2012) Mechanism of nucleotide sensing in group II chaperonins. EMBO J 31(3):731–740
Pereira JH, Ralston CY, Douglas NR, Meyer D, Knee KM, Goulet DR, King JA, Frydman J, Adams PD (2010) Crystal structures of a group II chaperonin reveal the open and closed states associated with the protein folding cycle. J Biol Chem 285(36):27958–27966
Reissmann S, Parnot C, Booth CR, Chiu W, Frydman J (2007) Essential function of the built-in lid in the allosteric regulation of eukaryotic and archaeal chaperonins. Nat Struct Mol Biol 14(5):432–440
Roobol A, Carden MJ (1999) Subunits of the eukaryotic cytosolic chaperonin CCT do not always behave as components of a uniform hetero-oligomeric particle. Eur J Cell Biol 78(1):21–32
Roobol A, Holmes FE, Hayes NV, Baines AJ, Carden MJ (1995) Cytoplasmic chaperonin complexes enter neurites developing in vitro and differ in subunit composition within single cells. J Cell Sci 108(Pt 4):1477–1488
Spiess C, Meyer AS, Reissmann S, Frydman J (2004) Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol 14(11):598–604
Spiess C, Miller EJ, McClellan AJ, Frydman J (2006) Identification of the TRiC/CCT substrate binding sites uncovers the function of subunit diversity in eukaryotic chaperonins. Mol Cell 24(1):25–37
Tam S, Geller R, Spiess C, Frydman J (2006) The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol 8(10):1155–1162
Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, Frydman J (2009) The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol 16(12):1279–1285
Tang Y-C, Chang H-C, Roeben A, Wischnewski D, Wischnewski N, Kerner MJ, Hartl FU, Hayer-Hartl M (2006) Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein. Cell 125(5):903–914
Thulasiraman V, Ferreyra RG, Frydman J (2000) Folding assays. Assessing the native conformation of proteins. Methods Mol Biol 140:169–177
Thulasiraman V, Yang CF, Frydman J (1999) In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J 18(1):85–95
Tran DP, Kim SJ, Park NJ, Jew TM, Martinson HG (2001) Mechanism of poly(A) signal transduction to RNA polymerase II in vitro. Mol Cell Biol 21(21):7495–7508
Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H (1992) TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature 358(6383):245–248
Zhang J, Baker ML, Schröder GF, Douglas NR, Reissmann S, Jakana J, Dougherty M, Fu CJ, Levitt M, Ludtke SJ, Frydman J, Chiu W (2010) Mechanism of folding chamber closure in a group II chaperonin. Nature 463(7279):379–383
Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC (2012) Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndrome protein complex, the BBSome. J Biol Chem 287(24):20625–20635
Acknowledgments
The authors thank Dr. Kate Moreau and Daniel Goulet for their helpful discussions. This work was funded by NIH Roadmap grant EY016525 and NEI grant EY015834.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knee, K.M., Sergeeva, O.A. & King, J.A. Human TRiC complex purified from HeLa cells contains all eight CCT subunits and is active in vitro. Cell Stress and Chaperones 18, 137–144 (2013). https://doi.org/10.1007/s12192-012-0357-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-012-0357-z