Abstract
Purpose
The Asian Pacific Association for the Study of the Liver (APASL) convened an international working party to develop a consensus cost-effectiveness model for treatment of Hepatitis B in Asia Pacific countries in March 2010.
Methods
The working party consisted of expert hepatologists, virologists and epidemiologists from 11 representative countries in the Asia Pacific region. Meetings were conducted at the 20th APASL Annual Meeting in 2010 to determine consensus estimates for modeling and at the 21st and 22nd APASL meetings in 2011 and 2012, respectively to review and approve the models.
Results
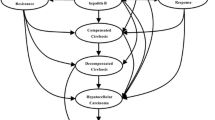
The consensus cost-effectiveness model used Singapore as base case analysis and was validated using actual data from the Singapore Cancer, Diseases and Death Registries. Simulation for Singapore, China, Thailand, Pakistan, Taiwan and Korea were performed. Antivirals with high resistance barriers like entecavir and tenofovir had the highest retail cost but were the most cost-effective therapy in developed countries such as Singapore, Taiwan and Korea while generic tenofovir was most cost effective in Thailand and Pakistan. The cost effectiveness of different treatment strategies varied significantly between countries and was affected by medication cost, economic affordability, access to liver transplantation and the prevailing health of the general population.
Conclusion
Choosing treatment strategies for hepatitis B based on low retail drug cost can be misleading because more expensive drugs may be more cost effective when considering long-term health outcomes and costs. Cost-effectiveness data should be individualized to countries based on their unique socio-economic conditions. Governmental policies which subsidize more costly drugs that have lower risk of drug resistance can benefit more patients.





Similar content being viewed by others
References
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatitis. 2004;11(2):97–107
Lesmana LALN-Y, Mahachai V, Phiet PH, Suh DJ, Yao GB, Hepatitis B. Overview of the burden of disease in the Asia-Pacific region. Liver Int. 2006;26(s2):3–10
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology (Baltimore, Md.) 2009;50(3):661–662. Epub 2009/08/29
European Association For The Study Of The L. EASL. Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50(2):227–242 Epub 2008/12/05
Liaw Y-F, Kao JH, Piratvisuth T, Chan HLY, Chien R-N, Liu C-J, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hep Int. 2012;6(3):531–561 Epub 17 May 2012
Sung JJ, Amarapurkar D, Chan HL, Cheng J, Kao JH, Han KH, et al. Treatment of chronic hepatitis B in Asia-Pacific countries: is the Asia-Pacific consensus statement being followed? Antivir Ther. 2010;15(4):607–616 Epub 2010/07/01
Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Ann Int Med. 2005;142(10):821–831
Dakin H, Bentley A, Dusheiko G. Cost-utility analysis of tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Value Health. 2010;13(8):922–933 Epub 2010/09/10
Veenstra DL, Sullivan SD, Clarke L, Iloeje UH, Tafesse E, Di Bisceglie A, et al. Cost effectiveness of entecavir versus Lamivudine with Adefovir salvage in HBeAg-positive chronic hepatitis B. PharmacoEconomics. 2007;25(11):963–977
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338
Keeffe EB, Zeuzem S, Koff RS, Dieterich DT, Esteban-Mur R, Gane EJ, et al. Report of an international workshop: roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5(8):890–897 Epub 2007/07/17
Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–686
Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011;141(4):1240–1248, 8 e1-2. Epub 2011/06/28
Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology (Baltimore, Md.). 2010;51(2):415–421 Epub 2009/11/11
Gane EJ, Marcellin P, Sievert W, Trinh HN, Shiffman ML, Washington MK, et al. Five years of treatment with Tenofovir Df (Tdf) for chronic hepatitis B (Chb) infection in Asian patients is associated with sustained viral suppression and significant regression of histological fibrosis and cirrhosis. Hepatology (Baltimore, Md.). 2011;54:1038a–1039a
National Wages and Productivity Commission P. Asian Wages. 2010 [cited 2012 05082012]; Available from: http://nwpc.dole.gov.ph/pages/statistics/Asean%20Wages%202010.pdf
Ong SC, Mak B, Aung MO, Li SC, Lim SG. Health-related quality of life in chronic hepatitis B patients. Hepatology (Baltimore, Md.). 2008;47(4):1108 Epub 2008/03/05
Shamliyan TA, MacDonald R, Shaukat A, Taylor BC, Yuan JM, Johnson JR, et al. Antiviral therapy for adults with chronic hepatitis B: a systematic review for a National Institutes of Health Consensus Development Conference. Ann Int Med. 2009;150(2):111–124 Epub 2009/01/07
WHO. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health: Executive Summary. World Health Organisation. 2001
Hong WW, Ang LW, Cutter JL, James L, Chew SK, Goh KT. Changing seroprevalence of hepatitis B virus markers of adults in Singapore. Ann Acad Med Singapore. 2010;39(8):591–598 Epub 2010/09/15
Statistics Do. Yearbook of Statitistics Singapore 1999
Registry SC. Cancer Registry Interim Report For The Period 2003–2007. 2009
Statistics Do. Yearbook of Statitistics Singapore 2009. 2009
Agency CI. The World Factbook. Country Comparison:Life Expectancy at Brith. 2011
Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139(2):491–498 Epub 2010/04/13
Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, et al. NIH consensus development statement on management of hepatitis B. NIH Consens State Sci Statements. 2008;25(2):1–29 Epub 2008/10/25
Dai CY, Tseng TC, Wong GL, Huang JF, Wong VW, Liu CJ, et al. Consolidation therapy for HBeAg-positive Asian chronic hepatitis B patients receiving lamivudine treatment: a multicentre study. J Antimicrob Chemother. 2013;68(10):2332–2338 Epub 2013/06/27
Compliance with ethical requirements and Conflict of interest
This article does not contain any studies with human or animal subjects. Dr. Dan Yock Young has received speaker honorariums and consultancy fees from Novartis, GSK, Gilead, Bristol Myers Squibb, MSD, Sanofi-Aventis and Roche. Dr. Kwang Hyub Han has received research grants and speaker honorarium from Sanofi Aventis. Dr. Ji Dong Jia has received speaker honorariums and consultancy fees from Novartis, Gilead, Bristol Myers Squibb, MSD, Boehringer Ingelheim and Roche. Dr. Chun Jen Liu has received speaker honorarium from Gilead, Bristol Myers Squibb, MSD and Roche. Dr. Teerha Piratvisuth has received research grants, speaker honorarium, and consultancy fees from Novartis, GSK, Bristol Myers Squibb, MSD and Roche. Dr. ASF Lok has served on advisory boards of Gilead, GlaxoSmithKline and Merck and has received research grants from Bristol-Myers Squibb, Gilead and Merck. Dr. Seng Gee Lim has received speaker honorariums and consultancy fees from Novartis, GSK, Gilead, Bristol Myers Squibb, MSD and Roche. Dr. Saeed Hamid and Dr JB Wong do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of APASL Hep B cost-effectiveness Working Group. The members of APASL Hep B cost-effectiveness Working Group are given in Appendix.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Henry Chan, Chinese University of Hong Kong, Hong Kong SAR China; Edward J. Gane, Auckland City Hospital, Auckland, New Zealand; Ding-Shinn Chen, National Taiwan University, Taiwan, Jia-Horng Kao, National Taiwan. University Hospital, Taiwan; Richard M. F. Yuen, University of Hong Kong, Hong Kong SAR, Osamu Yokosuka, Chiba University, Chiba, Japan, Geoffrey W. McCaughan. University of Sydney, Sydney, Australia; Stephen Locarnini, Royal Melbourne Hospital, Melbourne, Australia; Benjamin Cowie, Royal Melbourne Hospital, Melbourne. Australia.
Rights and permissions
About this article
Cite this article
Dan, Y.Y., Wong, J.B., Hamid, S.S. et al. Consensus cost-effectiveness model for treatment of chronic hepatitis B in Asia Pacific countries. Hepatol Int 8, 382–394 (2014). https://doi.org/10.1007/s12072-014-9549-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-014-9549-1