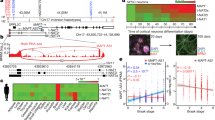
Abstract
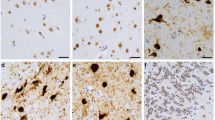
Microtubule binding protein Tau has been implicated in a wide range of neurodegenerative disorders collectively classified as tauopathies. Exon 10 of the human tau gene, which codes for a microtubule binding repeat region, is alternatively spliced to form Tau protein isoforms containing either four or three microtubule binding repeats, Tau4R and Tau3R, respectively. The levels of different Tau splicing isoforms are fine-tuned by alternative splicing with the ratio of Tau4R/Tau3R maintained approximately at one in adult neurons. Mutations that disrupt tau exon 10 splicing regulation cause an imbalance of different tau splicing isoforms and have been associated with tauopathy. To search for factors interacting with tau pre-messenger RNA (pre-mRNA) and regulating tau exon 10 alternative splicing, we performed a yeast RNA–protein interaction screen and identified polypyrimidine tract binding protein associated splicing factor (PSF) as a candidate tau exon 10 splicing regulator. UV crosslinking experiments show that PSF binds to the stem-loop structure at the 5′ splice site downstream of tau exon 10. This PSF-interacting RNA element is distinct from known PSF binding sites previously identified in other genes. Overexpression of PSF promotes tau exon 10 exclusion, whereas down-regulation of the endogenous PSF facilitates exon 10 inclusion. Immunostaining shows that PSF is expressed in the human brain regions affected by tauopathy. Our data reveal a new player in tau exon 10 alternative splicing regulation and uncover a previously unknown mechanism of PSF in regulating tau pre-mRNA splicing.







Similar content being viewed by others
References
Akhmedov AT, Lopez BS (2000) Human 100-kDa homologous DNA-pairing protein is the splicing factor PSF and promotes DNA strand invasion. Nucleic Acids Res 28(16):3022–3030
Andreadis A (2005) Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta 1739(2–3):91–103
Andreadis A (2006) Misregulation of tau alternative splicing in neurodegeneration and dementia. Prog Mol Subcell Biol 44:89–107
Andreadis A, Brown WM, Kosik KS (1992) Structure and novel exons of the human tau gene. Biochemistry 31(43):10626–10633
Antunes-Martins A, Mizuno K, Irvine EE, Lepicard EM, Giese KP (2007) Sex-dependent up-regulation of two splicing factors, Psf and Srp20, during hippocampal memory formation. Learn Mem 14:693–702
Black D (2000) Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell 103(3):367–370
Blanchette M, Chabot B (1997) A highly stable duplex structure sequesters the 5′ splice site region of hnRNP A1 alternative exon 7B. RNA 3(4):405–419
Boeve BF, Hutton M (2008) Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN). Arch Neurol 65(4):460–464
Broderick J, Wang J, Andreadis A (2004) Heterogeneous nuclear ribonucleoprotein E2 binds to tau exon 10 and moderately activates its splicing. Gene 331:107–114
Buratti E, Baralle FE (2004) Influence of RNA secondary structure on the pre-mRNA splicing process. Mol Cell Biol 24(24):10505–10514
Buxade M, Morrice N, Krebs DL, Proud CG (2008) The PSF.p54nrb complex is a novel Mnk substrate that binds the mRNA for tumor necrosis factor alpha. J Biol Chem 283(1):57–65
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM (2007) Consortium for frontotemporal lobar degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol 114(1):5–22
Chanas-Sacre G, Mazy-Servais C, Wattiez R, Pirard S, Rogister B, Patton JG, Belachew S, Malgrange B, Moonen G, Leprince P (1999) Identification of PSF, the polypyrimidine tract-binding protein-associated splicing factor, as a developmentally regulated neuronal protein. J Neurosci Res 57(1):62–73
Clouet d’Orval B, d’Aubenton Carafa Y, Sirand-Pugnet P, Gallego M, Brody E, Marie J (1991) RNA secondary structure repression of a muscle-specific exon in HeLa cell nuclear extracts. Science 252(5014):1823–1828
Cobbold LC, Spriggs KA, Haines SJ, Dobbyn HC, Hayes C, de Moor CH, Lilley KS, Bushell M, Willis AE (2008) Identification of internal ribosome entry segment (IRES)-trans-acting factors for the Myc family of IRESs. Mol Cell Biol 28(1):40–49
Collet J, Fehrat L, Pollard H, Ribas de Pouplana L, Charton G, Bernard A, Moreau J, Ben-Ari Y, Khrestchatisky M (1997) Developmentally regulated alternative splicing of mRNAs encoding N-terminal tau variants in the rat hippocampus: structural and functional implications. Eur J Neurosci 9:2723–2733
Colombo R, Tavian D, Baker MC, Richardson AM, Snowden JS, Neary D, Mann DM, Pickering-Brown SM (2009) Recent origin and spread of a common Welsh MAPT splice mutation causing frontotemporal lobar degeneration. Neurogenetics 10(4):313–318
Connell JW, Gibb GM, Betts JC, Blackstock WP, Gallo J, Lovestone S, Hutton M, Anderton BH (2001) Effects of FTDP-17 mutations on the in vitro phosphorylation of tau by glycogen synthase kinase 3beta identified by mass spectrometry demonstrate certain mutations exert long-range conformational changes. FEBS Lett 493:40–44
Cooper TA, Wan L, Dreyfuss G (2009) RNA and disease. Cell 136(4):777–793
Dawson HN, Cantillana V, Chen L, Vitek MP (2007) The tau N279K exon 10 splicing mutation recapitulates frontotemporal dementia and parkinsonism linked to chromosome 17 tauopathy in a mouse model. J Neurosci 27(34):9155–9168
Donahue CP, Muratore C, Wu JY, Kosik KS, Wolfe MS (2006) Stabilization of the tau exon 10 stem loop alters pre-mRNA splicing. J Biol Chem 281(33):23302–23306
D’Souza I, Schellenberg GD (2000) Determinants of 4-repeat tau expression. Coordination between enhancing and inhibitory splicing sequences for exon 10 inclusion. J Biol Chem 275(23):17700–17709
Duong HA, Robles MS, Knutti D, Weitz CJ (2011) A molecular mechanism for circadian clock negative feedback. Science 332(6036):1436–1439
Eperon LP, IR Graham, AD Griffiths and IC Eperon (1988) Effects of RNA secondary structure on alternative splicing of pre-mRNA: is folding limited to a region behind the transcribing RNA polymerase? Cell 54:393–401
Gao QS, Memmott J, Lafyatis R, Stamm S, Screaton G, Andreadis A (2000) Complex regulation of tau exon 10, whose missplicing causes frontotemporal dementia. J Neurochem 74(2):490–500
Gao L, Wang J, Wang Y, Andreadis A (2007) SR protein 9G8 modulates splicing of tau exon 10 via its proximal downstream intron, a clustering region for frontotemporal dementia mutations. Mol Cell Neurosci 34(1):48–58
Glatz DC, Rujescu D, Tang Y, Berendt FJ, Hartmann AM, Faltraco F, Rosenberg C, Hulette C, Jellinger K, Hampel H, Riederer P, Moller HJ, Andreadis A, Henkel K, Stamm S (2006) The alternative splicing of tau exon 10 and its regulatory proteins CLK2 and TRA2-BETA1 changes in sporadic Alzheimer’s disease. J Neurochem 96(3):635–644
Goedert M, Jakes R (1990) Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 9(13):4225–4230
Goedert M, Jakes R (2005) Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta 1739(2–3):240–250
Goedert M, Spillantini MG (2001) Tau gene mutations and neurodegeneration. Biochem Soc Symp (67):59–71
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989a) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3(4):519–526
Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA (1989b) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 8(2):393–399
Goode BL, Chau M, Denis PE, Feinstein SC (2000) Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J Biol Chem 275:38182–38189
Gozani O, Patton JG, Reed R (1994) A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. EMBO J 13(14):3356–3367
Grant RC, Harwood SJ, Wells RD (1968) The synthesis and characterization of poly d (I-C) poly d (I-C). J Am Chem Soc 90(16):4474–4476
Greco-Stewart VS, Thibault CS, Pelchat M (2006) Binding of the polypyrimidine tract-binding protein-associated splicing factor (PSF) to the hepatitis delta virus RNA. Virology 356(1–2):35–44
Grover A, Houlden H, Baker M, Adamson J, Lewis J, Prihar G, Pickering-Brown S, Duff K, Hutton M (1999) 5′ Splice site mutations in tau associated with the inherited dementia FTDP-17 affect a stem-loop structure that regulates alternative splicing of exon 10. J Biol Chem 274(21):15134–15143
Hartmann M, Rujescu D, Giannakouros T, Nikolakaki E, Goedert M, Mandelkow EM, Gao QS, Andreadis A, Stamm S (2001) Regulation of alternative splicing of human tau exon 10 by phosphorylation of splicing factors. Mol Cell Neurosci 18(1):80–90
Hasegawa M, Smith MJ, Goedert M (1998) Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 437:207–210
Hasegawa M, Smith MJ, Iijima M, Tabira T, Goedert M (1999) FTDP-17 mutations N279K and S305N in tau produce increased splicing of exon 10. FEBS Lett 443:93–96
Himmler A (1989) Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol 9:1389–1396
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393(6686):702–705
Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118(1):53–69
Iqbal K, F Liu, CX Gong, I Grundke-Iqbal (2010). Tau in Alzheimer Disease and related Tauopathies. Curr Alzheimer Res 7:656–664.
Jiang ZH, Zhang WJ, Rao Y, Wu JY (1998) Regulation of Ich-1 pre-mRNA alternative splicing and apoptosis by mammalian splicing factors. Proc Natl Acad Sci USA 95(16):9155–9160
Jiang Z, Cote J, Kwon JM, Goate AM, Wu JY (2000) Aberrant splicing of tau pre-mRNA caused by intronic mutations associated with the inherited dementia frontotemporal dementia with parkinsonism linked to chromosome 17. Mol Cell Biol 20(11):4036–4048
Jiang Z, Tang H, Havlioglu N, Zhang X, Stamm S, Yan R, Wu JY (2003) Mutations in tau gene exon 10 associated with FTDP-17 alter the activity of an exonic splicing enhancer to interact with Tra2 beta. J Biol Chem 278(21):18997–19007
Kalbfuss B, Mason SA, Misteli T (2001) Correction of alternative splicing of tau in frontotemporal dementia and parkinsonism linked to chromosome 17. J Biol Chem 276(46):42986–42993
Kameoka S, Duque P, Konarska MM (2004) P54(nrb) associates with the 5′ splice site within large transcription/splicing complexes. EMBO J 23(8):1782–1791
Kaneko S, Rozenblatt-Rosen O, Meyerson M, Manley JL (2007) The multifunctional protein p54nrb/PSF recruits the exonuclease XRN2 to facilitate pre-mRNA 3′ processing and transcription termination. Genes Dev 21(14):1779–1789
Kar A, Kuo D, He R, Zhou J, Wu JY (2005) Tau alternative splicing and frontotemporal dementia. Alzheimer Dis Assoc Disord 19(Suppl 1):S29–S36
Kar A, Havlioglu N, Tarn WY, Wu JY (2006) RBM4 interacts with an intronic element and stimulates tau exon 10 inclusion. J Biol Chem 281(34):24479–24488
Kar A, Fushimi K, Zhou Xh, Ray P, Shi C, Chen Xp, Liu Zr, Chen S, Wu JY (2011) RNA Helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5′ splice site. Mol Cell Biol 31:1812–1821
Kondo S, Yamamoto N, Murakami T, Okumura M, Mayeda A, Imaizumi K (2004) Tra2 beta, SF2/ASF and SRp30c modulate the function of an exonic splicing enhancer in exon 10 of tau pre-mRNA. Genes Cells 9:121–130
Konzack S, Thies E, Marx A, Mandelkow EM, Mandelkow E (2007) Swimming against the tide: mobility of the microtubule-associated protein tau in neurons. J Neurosci 27:9916–9927
Krawczak M, Reiss J, Cooper DN (1992) The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet 90(1–2):41–54
LeBoeuf AC, Levy SF, Gaylord M, Bhattacharya A, Singh AK, Jordan MA, Wilson L, Feinstein SC (2008) FTDP-17 mutations in Tau alter the regulation of microtubule dynamics: an "alternative core" model for normal and pathological Tau action. J Biol Chem 283:36406–36415
Lee G, Neve RL, Kosik KS (1989) The microtubule binding domain of tau protein. Neuron 2:1615–1624
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Libri D, Piseri A, Fiszman MY (1991) Tissue-specific splicing in vivo of the beta-tropomyosin gene: dependence on an RNA secondary structure. Science 252(5014):1842–1845
Lichtenberg-Kraag B, Mandelkow EM (1990) Isoforms of tau protein from mammalian brain and avian erythrocytes: structure, self-assembly, and elasticity. J Struct Biol 105:46–53
Liu F, Gong CX (2008) Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener 3:8
Lowery LA, Rubin J, Sive H (2007) Whitesnake/sfpq is required for cell survival and neuronal development in the zebrafish. Dev Dyn 236(5):1347–1357
Mackenzie IR, Rademakers R (2007) The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenet Nov 8(4):237–248
Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119(1):1–4
Mathur M, Tucker PW, Samuels HH (2001) PSF is a novel corepressor that mediates its effect through Sin3A and the DNA binding domain of nuclear hormone receptors. Mol Cell Biol 21(7):2298–2311
Matlin AJ, Clark F, Smith CW (2005) Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol 6:386–398
Matsuo M, Nishio H, Kitoh Y, Francke U, Nakamura H (1992) Partial deletion of a dystrophin gene leads to exon skipping and to loss of an intra-exon hairpin structure from the predicted mRNA precursor. Biochem Biophys Res Commun 182(2):495–500
Medeiros R, Baglietto-Vargas D, Laferla FM (2010) The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther. doi:10.1111/j.1755-5949.2010.00177.x
Melton AA, Jackson J, Wang J, Lynch KW (2007) Combinatorial control of signal-induced exon repression by hnRNP L and PSF. Mol Cell Biol 27(19):6972–6984
Mercken M, Fischer I, Kosik KS, Nixon RA (1995) Three distinct axonal transport rates for tau, tubulin, and microtubule-associated proteins: evidence for dynamic interactions of tau with microtubules in vivo. J Neurosci 15(12):8259–8267
Montejo de Garcini E, Corrochano L, Wischik CM, Diaz Nido J, Correas I, Avila J (1992) Differentiation of neuroblastoma cells correlates with an altered splicing pattern of tau RNA. FEBS Lett 299:10–14
Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST (2009) Axonal transport defects in neurodegenerative diseases. J Neurosci 29(41):12776–12786
Neumann M, Tolnay M, Mackenzie IR (2009) The molecular basis of frontotemporal dementia. Expert Rev Mol Med 11:e23
Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA (1986) Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 387(3):271–280
Nilsen TW, Graveley BR (2010) Expansion of the eukaryotic proteome by alternative splicing. Nature 463(7280):457–463
Panda D, Goode BL, Feinstein SC, Wilson L (1995) Kinetic stabilization of microtubule dynamics at steady state by tau and microtubule-binding domains of tau. Biochemistry 34(35):11117–11127
Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B (1993) Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev 7(3):393–406
Peng R, Dye BT, Perez I, Barnard DC, Thompson AB, Patton JG (2002) PSF and p54nrb bind a conserved stem in U5 snRNA. RNA 8(10):1334–1347
Pickering-Brown S, Baker M, Yen SH, Liu WK, Hasegawa M, Cairns N, Lantos PL, Rossor M, Iwatsubo T, Davies Y, Allsop D, Furlong R, Owen F, Hardy J, Mann D, Hutton M (2000) Pick’s disease is associated with mutations in the tau gene. Ann Neurol 48(6):859–867
Pickering-Brown SM, Richardson AM, Snowden JS, McDonagh AM, Burns A, Braude W, Baker M, Liu WK, Yen SH, Hardy J, Hutton M, Davies Y, Allsop D, Craufurd D, Neary D, Mann DM (2002) Inherited frontotemporal dementia in nine British families associated with intronic mutations in the tau gene. Brain 125(Pt 4):732–751
Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR (2002) Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54(nrb)/NonO, protein-associated splicing factor, and SF-1, a complex that also participates in repression of transcription. Endocrinology 143(4):1280–1290
Shav-Tal Y, Zipori D (2002) PSF and p54(nrb)/NonO—multi-functional nuclear proteins. FEBS Lett 531(2):109–114
Singh NN, Singh RN, Androphy EJ (2007) Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. Nucleic Acids Res 35(2):371–389
Sirand-Pugnet P, Durosay P, Clouet d’Orval BC, Brody E, Marie J (1995) Beta-tropomyosin pre-mRNA folding around a muscle-specific exon interferes with several steps of spliceosome assembly. J Mol Biol 251(5):591–602
Solis AS, Shariat N, Patton JG (2008) Splicing fidelity, enhancers, and disease. Front Biosci 13:1926–1942
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 95:7737–7741
Spillantini MG, Van Swieten JC, Goedert M (2000) Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Neurogenetics 2(4):193–205
Straub T, Grue P, Uhse A, Lisby M, Knudsen BR, Tange TO, Westergaard O, Boege F (1998) The RNA-splicing factor PSF/p54 controls DNA-topoisomerase I activity by a direct interaction. J Biol Chem 273(41):26261–26264
Tomoo K, Yao TM, Minoura K, Hiraoka S, Sumida M, Taniguchi T, Ishida T (2005) Possible role of each repeat structure of the microtubule-binding domain of the tau protein in in vitro aggregation. J Biochem 138:413–423
Urban RJ, Bodenburg Y, Kurosky A, Wood TG, Gasic S (2000) Polypyrimidine tract-binding protein-associated splicing factor is a negative regulator of transcriptional activity of the porcine p450scc insulin-like growth factor response element. Mol Endocrinol 14(6):774–782
Utton MA, Connell J, Asuni AA, van Slegtenhorst M, Hutton M, de Silva R, Lees AJ, Miller CC, Anderton BH (2002) The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons. J Neurosci 22:6394–6400
Varani L, Hasegawa M, Spillantini MG, Smith MJ, Murrell JR, Ghetti B, Klug A, Goedert M, Varani G (1999) Structure of tau exon 10 splicing regulatory element RNA and destabilization by mutations of frontotemporal dementia and parkinsonism linked to chromosome 17. Proc Natl Acad Sci USA 96:8229–8234
Varani L, Spillantini MG, Goedert M, Varani G (2000) Structural basis for recognition of the RNA major groove in the tau exon 10 splicing regulatory element by aminoglycoside antibiotics. Nucleic Acids Res 28:710–719
Wang GS, Cooper TA (2007) Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet 8(10):749–761
Wang J, Gao QS, Wang Y, Lafyatis R, Stamm S, Andreadis A (2004) Tau exon 10, whose missplicing causes frontotemporal dementia, is regulated by an intricate interplay of cis elements and trans factors. J Neurochem 88(5):1078–1090
Wang G, Cui Y, Zhang G, Garen A, Song X (2009) Regulation of proto-oncogene transcription, cell proliferation, and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA. Proc Natl Acad Sci 106(39):16794–16798
Wang Y, Gao L, Tse SW, Andreadis A (2010) Heterogeneous nuclear ribonucleoprotein E3 modestly activates splicing of tau exon 10 via its proximal downstream intron, a hotspot for frontotemporal dementia mutations. Gene 451(1–2):23–31
Ward AJ, Cooper TA (2010) The pathobiology of splicing. J Pathol 220(2):152–163
Wei ML, Andreadis A (1998) Splicing of a regulated exon reveals additional complexity in the axonal microtubule-associated protein tau. J Neurochem 70:1346–1356
Wei ML, Memmott J, Screaton G, Andreadis A (2000) The splicing determinants of a regulated exon in the axonal MAP tau reside within the exon and in its upstream intron. Brain Res Mol Brain Res 80(2):207–218
Wolfe MS (2009) Tau mutations in neurodegenerative diseases. J Biol Chem 284(10):6021–6025
Wu JY, Postashkin JA (2009) Alternative splicing in the nervous system. In: Squire LR (ed) Encyclopedia of neuroscience, vol 1. Academic/Elsevier, Oxford, pp 245–251
Wu JY, Yuan L, Havlioglu N (2004) Alternatively spliced genes. In: Meyers RA (ed) Encyclopedia of molecular cell biology and molecular medicine, vol 1, 2nd edn. Wiley-VCH, Chichester, 125–177
Wu JY, Kar A, Kuo D, Yu B, Havlioglu N (2006) SRp54 (SFRS11), a regulator for tau exon 10 alternative splicing identified by an expression cloning strategy. Mol Cell Biol 26(18):6739–6747
Yang Z, Sui Y, Xiong S, Liour SS, Phillips AC, Ko L (2007) Switched alternative splicing of oncogene CoAA during embryonal carcinoma stem cell differentiation. Nucleic Acids Res 35(6):1919–1932
Yu Q, Guo J, Zhou J (2004) A minimal length between tau exon 10 and 11 is required for correct splicing of exon 10. J Neurochem 90(1):164–172
Zhang Z, Carmichael GG (2001) The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell 106(4):465–475
Zhou J, Yu Q, Zou T (2008) Alternative splicing of exon 10 in the tau gene as a target for treatment of tauopathies. BMC Neurosci 9(Suppl 2):S10
Zychlinski D, Erkelenz S et al (2009) Limited complementarity between U1 snRNA and a retroviral 5′ splice site permits its attenuation via RNA secondary structure. Nucleic Acids Res 37(22):7429–7440
Acknowledgment
We are grateful for generous help from Dr. James Patton (Vanderbilt University) and Dr. Douglas Black (UCLA) for various reagents. We thank members of the Wu laboratory for helpful discussions, critical reading and suggestions. This work was supported by National Institutes of Health (R01 AG033004 and R56NS074763 to JYW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ray, P., Kar, A., Fushimi, K. et al. PSF Suppresses Tau Exon 10 Inclusion by Interacting with a Stem-Loop Structure Downstream of Exon 10. J Mol Neurosci 45, 453–466 (2011). https://doi.org/10.1007/s12031-011-9634-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9634-z