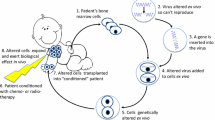
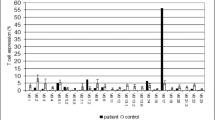
Abstract
Rapid progress has been made from the identification of the molecular defects causing X-linked severe combined immune deficiency (X-SCID) to the development of cutting-edge therapeutic approaches such as hematopoietic stem cell transplant and gene therapy for XSCID. Successful treatment of XSCID has created a new population of patients, many of whom are now adolescents and young adults and are facing a variety of chronic problems secondary to partial correction of their underlying disease. This review focuses on the clinical challenges facing these patients (and their caregivers) and provides an overview of some of the treatment options available, including gene therapy.




Similar content being viewed by others
References
Glanzmann E, Riniker P. Essential lymphocytophthisis; new clinical aspect of infant pathology. Ann Paediatr. 1950;175(1–2):1–32.
Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–87.
Fischer A, Le Deist F, Hacein-Bey-Abina S, Andre-Schmutz I, Basile Gde S, de Villartay JP, et al. Severe combined immunodeficiency. A model disease for molecular immunology and therapy. Immunol Rev. 2005;203:98–109.
Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55.
Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–57.
Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ, Willard HF, et al. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum Mol Genet. 1993;2(8):1099–104.
Huang LH, Shyur SD, Weng JD, Shin C, Huang FY, Tzen CY. Disseminated cutaneous bacille Calmette-Guerin infection identified by polymerase chain reaction in a patient with X-linked severe combined immunodeficiency. Pediatr Dermatol. 2006;23(6):560–3.
Huang LH, Shyur SD, Weng JD, Shin C, Tzen CY, Huang FY. Disseminated Bacille Calmette-Guerin disease as the initial presentation of X-linked severe combined immunodeficiency—a case report. Asian Pac J Allergy Immunol. 2005;23(4):221–6.
Denianke KS, Frieden IJ, Cowan MJ, Williams ML, McCalmont TH. Cutaneous manifestations of maternal engraftment in patients with severe combined immunodeficiency: a clinicopathologic study. Bone Marrow Transplant. 2001;28(3):227–33.
Muller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood. 2001;98(6):1847–51.
Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8.
Puck JM. Neonatal screening for severe combined immune deficiency. Curr Opin Allergy Clin Immunol. 2007;7(6):522–7.
Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2(7583):1366–9.
Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–16.
Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361(9357):553–60.
Somech R, Roifman CM. Mutation analysis should be performed to rule out gammac deficiency in children with functional severe combined immune deficiency despite apparently normal immunologic tests. J Pediatr. 2005;147(4):555–7.
Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997;94(7):3168–71.
Chinen J, Davis J, De Ravin SS, Hay BN, Hsu AP, Linton GF, et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood. 2007;110(1):67–73.
Muller-Ruchholtz W, Wottge HU, Muller-Hermelink HK. Lymphocytes and hemopoietic bone marrow cells—antigenic relationships between these cells and lymphatic tissue repopulation from stem cells. Adv Exp Med Biol. 1976;66:147–52.
Speckmann C, Pannicke U, Wiech E, Schwarz K, Fisch P, Friedrich W, et al. Clinical and immunological consequences of a somatic reversion in a patient with X-linked severe combined immunodeficiency. Blood. 2008; Aug 26 [Epub ahead of print].
Wada T, Yasui M, Toma T, Nakayama Y, Nishida M, Shimizu M, et al. Detection of T lymphocytes with a second-site mutation in skin lesions of atypical X-linked severe combined immunodeficiency mimicking Omenn syndrome. Blood. 2008;112(5):1872–5.
Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322.
Leonard WJ. The molecular basis of X-linked severe combined immunodeficiency: defective cytokine receptor signaling. Annu Rev Med. 1996;47:229–39.
Felsburg PJ, Hartnett BJ, Henthorn PS, Moore PF, Krakowka S, Ochs HD. Canine X-linked severe combined immunodeficiency. Vet Immunol Immunopathol. 1999;69(2–4):127–35.
Felsburg PJ, Somberg RL, Perryman LE. Domestic animal models of severe combined immunodeficiency: canine X-linked severe combined immunodeficiency and severe combined immunodeficiency in horses. Immunodefic Rev. 1992;3(4):277–303.
Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2(3):223–38.
Hong R, Cooper MD, Allan MJ, Kay HE, Meuwissen H, Good RA. Immunological restitution in lymphopenic immunological deficiency syndrome. Lancet. 1968;1(7541):503–6.
Simonsen M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349–467.
Bortin MM, Rimm AA. Severe combined immunodeficiency disease: characterization of the disease and results of transplantation. Transplant Proc. 1977;9(1):169–70.
Rimm AA, Bortin MM. HLA antigens and severe combined immunodeficiency disease. Lancet. 1977;1(8026):1361–2.
Moen RC, Horowitz SD, Sondel PM, Borcherding WR, Trigg ME, Billing R, et al. Immunologic reconstitution after haploidentical bone marrow transplantation for immune deficiency disorders: treatment of bone marrow cells with monoclonal antibody CT-2 and complement. Blood. 1987;70(3):664–9.
Giri N, Vowels M, Ziegler JB, Ford D, Lam-Po-Tang R. HLA non-identical T-cell-depleted bone marrow transplantation for primary immunodeficiency diseases. Aust N Z J Med. 1994;24(1):26–30.
Reisner Y. Differential agglutination by soybean agglutinin of human leukemia and neuroblastoma cell lines: potential application to autologous bone marrow transplantation. Proc Natl Acad Sci USA. 1983;80(21):6657–61.
Friedrich W, Goldmann SF, Ebell W, Blutters-Sawatzki R, Gaedicke G, Raghavachar A, et al. Severe combined immunodeficiency: treatment by bone marrow transplantation in 15 infants using HLA-haploidentical donors. Eur J Pediatr. 1985;144(2):125–30.
Buckley RH, Schiff SE, Sampson HA, Schiff RI, Markert ML, Knutsen AP, et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986;136(7):2398–407.
Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B, et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61(2):341–8.
Roifman CM, Grunebaum E, Dalal I, Notarangelo L. Matched unrelated bone marrow transplant for severe combined immunodeficiency. Immunol Res. 2007;38(1–3):191–200.
Dvorak CC, Cowan MJ. Hematopoietic stem cell transplantation for primary immunodeficiency disease. Bone Marrow Transplant. 2008;41(2):119–26.
Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91(10):3646–53.
Haddad E, Le Deist F, Aucouturier P, Cavazzana-Calvo M, Blanche S, De Saint Basile G, et al. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: a single-center study of 22 patients. Blood. 1999;94(8):2923–30.
Buckley RH. Advances in the understanding and treatment of human severe combined immunodeficiency. Immunol Res. 2000;22(2–3):237–51.
Fischer A, Landais P, Friedrich W, Morgan G, Gerritsen B, Fasth A, et al. European experience of bone-marrow transplantation for severe combined immunodeficiency. Lancet. 1990;336(8719):850–4.
Friedrich W, Honig M, Muller SM. Long-term follow-up in patients with severe combined immunodeficiency treated by bone marrow transplantation. Immunol Res. 2007;38(1–3):165–73.
Patel DD, Gooding ME, Parrott RE, Curtis KM, Haynes BF, Buckley RH. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 2000;342(18):1325–32.
Slatter MA, Bhattacharya A, Abinun M, Flood TJ, Cant AJ, Gennery AR. Outcome of boost haemopoietic stem cell transplant for decreased donor chimerism or graft dysfunction in primary immunodeficiency. Bone Marrow Transplant. 2005;35(7):683–9.
Slatter MA, Gennery AR. Umbilical cord stem cell transplantation for primary immunodeficiencies. Expert Opin Biol Ther. 2006;6(6):555–65.
Moss PA, Moots RJ, Rosenberg WM, Rowland-Jones SJ, Bodmer HC, McMichael AJ, et al. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci USA. 1991;88(20):8987–90.
Sarzotti M, Patel DD, Li X, Ozaki DA, Cao S, Langdon S, et al. T cell repertoire development in humans with SCID after nonablative allogeneic marrow transplantation. J Immunol. 2003;170(5):2711–8.
Cavazzana-Calvo M, Carlier F, Le Deist F, Morillon E, Taupin P, Gautier D, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109(10):4575–81.
Thrasher AJ, Hacein-Bey-Abina S, Gaspar HB, Blanche S, Davies EG, Parsley K, et al. Failure of SCID-X1 gene therapy in older patients. Blood. 2005;105(11):4255–7.
Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173(3):1604–11.
Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31(12):1800–12.
Hussain N, Quezado M, Huizing M, Geho D, White JG, Gahl W, et al. Intestinal disease in Hermansky-Pudlak syndrome: occurrence of colitis and relation to genotype. Clin Gastroenterol Hepatol. 2006;4(1):73–80.
Kouklakis G, Efremidou EI, Papageorgiou MS, Pavlidou E, Manolas KJ, Liratzopoulos N. Complicated Crohn’s-like colitis, associated with Hermansky-Pudlak syndrome, treated with Infliximab: a case report and brief review of the literature. J Med Case Reports. 2007;1:176 [PubMed-in process].
Adriani M, Garbi C, Amodio G, Russo I, Giovannini M, Amorosi S, et al. Functional interaction of common gamma-chain and growth hormone receptor signaling apparatus. J Immunol. 2006;177(10):6889–95.
Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-I generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86(11):5176–83.
De Ravin SS. Short stature in partially corrected X-linked severe combined immunodeficiency-suboptimal response to growth hormone. J Pediatr Endocrinol Metab. 2008 (in press).
Salerno M, Busiello R, Esposito V, Cosentini E, Adriani M, Selleri C, et al. Allogeneic bone marrow transplantation restores IGF-I production and linear growth in a gamma-SCID patient with abnormal growth hormone receptor signaling. Bone Marrow Transplant. 2004;33(7):773–5.
Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–6.
Boivin G, Goyette N, Bernatchez H. Prolonged excretion of amantadine-resistant influenza a virus quasi species after cessation of antiviral therapy in an immunocompromised patient. Clin Infect Dis. 2002;34(5):E23–5.
Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003;348(9):867–8.
Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J Infect Dis. 2006;193(6):760–4.
Laffort C, Le Deist F, Favre M, Caillat-Zucman S, Radford-Weiss I, Debre M, et al. Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common gammac cytokine receptor subunit or JAK-3 deficiency. Lancet. 2004;363(9426):2051–4.
Henthorn PS, Somberg RL, Fimiani VM, Puck JM, Patterson DF, Felsburg PJ. IL-2R gamma gene microdeletion demonstrates that canine X-linked severe combined immunodeficiency is a homologue of the human disease. Genomics. 1994;23(1):69–74.
Goldschmidt MH, Kennedy JS, Kennedy DR, Yuan H, Holt DE, Casal ML, et al. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J Virol. 2006;80(13):6621–8.
Nishio H, Matsui K, Tsuji H, Tamura A, Suzuki K. Immunolocalisation of the janus kinases (JAK)—signal transducers and activators of transcription (STAT) pathway in human epidermis. J Anat. 2001;198(Pt 5):581–9.
Ratjen F, Rjabko O, Kremens B. High-dose corticosteroid therapy for bronchiolitis obliterans after bone marrow transplantation in children. Bone Marrow Transplant. 2005;36(2):135–8.
Fischer A, Hacein-Bey-Abina S, Lagresle C, Garrigue A, Cavazana-Calvo M. Gene therapy of severe combined immunodeficiency disease: proof of principle of efficiency and safety issues. Gene therapy, primary immunodeficiencies, retrovirus, lentivirus, genome. Bull Acad Natl Med. 2005;189(5):779–85. Discussion 786–8.
Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346(16):1185–93.
Baum C, Schambach A, Modlich U, Thrasher A. Gene therapy of SCID-X1. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50(12):1507–17.
Cavazzana-Calvo M, Fischer A. Gene therapy for severe combined immunodeficiency: are we there yet? J Clin Invest. 2007;117(6):1456–65.
Qasim W, Gaspar HB, Thrasher AJ. Update on clinical gene therapy in childhood. Arch Dis Child. 2007;92(11):1028–31.
Santilli G, Thornhill SI, Kinnon C, Thrasher AJ. Gene therapy of inherited immunodeficiencies. Expert Opin Biol Ther. 2008;8(4):397–407.
Sokolic R, Kesserwan C, Candotti F. Recent advances in gene therapy for severe congenital immunodeficiency diseases. Curr Opin Hematol. 2008;15(4):375–80.
Gaspar HB, Howe S, Thrasher AJ. Gene therapy progress and prospects: gene therapy for severe combined immunodeficiency. Gene Ther. 2003;10(24):1999–2004.
Aviles Mendoza GJ, Seidel NE, Otsu M, Anderson SM, Simon-Stoos K, Herrera A, et al. Comparison of five retrovirus vectors containing the human IL-2 receptor gamma chain gene for their ability to restore T and B lymphocytes in the X-linked severe combined immunodeficiency mouse model. Mol Ther. 2001;3(4):565–73.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348(3):255–6.
Cavazzana-Calvo M, Lagresle C, Hacein-Bey-Abina S, Fischer A. Gene therapy for severe combined immunodeficiency. Annu Rev Med. 2005;56:585–602.
Fischer A, Abina SH, Thrasher A, von Kalle C, Cavazzana-Calvo M. LMO2 and gene therapy for severe combined immunodeficiency. N Engl J Med. 2004;350(24):2526–7. Author reply 2526–7.
Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143–50.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–9.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–9.
Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300(5626):1749–51.
Hematti P, Hong BK, Ferguson C, Adler R, Hanawa H, Sellers S, et al. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2004;2(12):e423.
Modlich U, Bohne J, Schmidt M, von Kalle C, Knoss S, Schambach A, et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108(8):2545–53.
Moreb JS, Schweder M. Human A1, a Bcl-2-related gene, is induced in leukemic cells by cytokines as well as differentiating factors. Leukemia. 1997;11(7):998–1004.
Du Y, Jenkins NA, Copeland NG. Insertional mutagenesis identifies genes that promote the immortalization of primary bone marrow progenitor cells. Blood. 2005;106(12):3932–9.
Rabbitts TH, Bucher K, Chung G, Grutz G, Warren A, Yamada Y. The effect of chromosomal translocations in acute leukemias: the LMO2 paradigm in transcription and development. Cancer Res. 1999;59(7 Suppl):1794s–8s.
Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92(15):6733–7.
Dave UP, Jenkins NA, Copeland NG. Gene therapy insertional mutagenesis insights. Science. 2004;303(5656):333.
Nienhuis AW, Dunbar CE, Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13(6):1031–49.
Thornhill SI, Schambach A, Howe SJ, Ulaganathan M, Grassman E, Williams D, et al. Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency. Mol Ther. 2008;16(3):590–8.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–96.
Felsenfeld G, Burgess-Beusse B, Farrell C, Gaszner M, Ghirlando R, Huang S, et al. Chromatin boundaries and chromatin domains. Cold Spring Harb Symp Quant Biol. 2004;69:245–50.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–7.
Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72(12):9873–80.
Schambach A, Bohne J, Chandra S, Will E, Margison GP, Williams DA, et al. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol Ther. 2006;13(2):391–400.
West AG, Gaszner M, Felsenfeld G. Insulators: many functions, many mechanisms. Genes Dev. 2002;16(3):271–88.
Trobridge GD, Miller DG, Jacobs MA, Allen JM, Kiem HP, Kaul R, et al. Foamy virus vector integration sites in normal human cells. Proc Natl Acad Sci USA. 2006;103(5):1498–503.
De Palma M, Montini E, Santoni de Sio FR, Benedicenti F, Gentile A, Medico E. Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells. Blood. 2005;105(6):2307–15.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72(11):8463–71.
Yu SF, von Ruden T, Kantoff PW, Garber C, Seiberg M, Ruther U, et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83(10):3194–8.
Modlich U, Kustikova OS, Schmidt M, Rudolph C, Meyer J, Li Z, et al. Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood. 2005;105(11):4235–46.
Naumann N, De Ravin SS, Choi U, Moayeri M, Whiting-Theobald N, Linton GF, et al. Simian immunodeficiency virus lentivector corrects human X-linked chronic granulomatous disease in the NOD/SCID mouse xenograft. Gene Ther. 2007;14(21):1513–24.
Puthenveetil G, Scholes J, Carbonell D, Qureshi N, Xia P, Zeng L, et al. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104(12):3445–53.
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2008;16(4):718–25 [Epub 2008].
Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93(21):11382–8.
Cartier N. Gene therapy strategies for X-linked adrenoleukodystrophy. Curr Opin Mol Ther. 2001;3(4):357–61.
Cartier N. Preliminary data from the first hematopoietic stem cell gene therapy trial with lentiviral vector demonstrate expression of the therapeutic protein in high percentage of lymphocytes and monocytes in two patients with X-linked adrenoleukodystrophy. Hum Gene Ther. 2007;18(10):941–53.
http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.
Acknowledgment
This project was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases. We thank Dr Kol Zarember for his critical review of the manuscript and apologize to the authors of many excellent publications that we were not able to present adequately in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Ravin, S.S., Malech, H.L. Partially corrected X-linked severe combined immunodeficiency: long-term problems and treatment options. Immunol Res 43, 223–242 (2009). https://doi.org/10.1007/s12026-008-8073-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-008-8073-6