Abstract
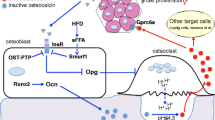
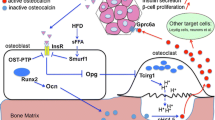
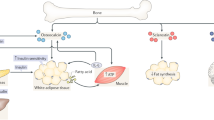
Diseases of compromised glucose metabolism, such as type II diabetes, have been for years correlated with defects in bone mass or strength. However, a reverse regulation of energy homeostasis by the skeleton was not suspected until very recently. A series of genetic studies have demonstrated that the skeleton regulates glucose metabolism and energy expenditure in an endocrine manner. The bone-derived protein osteocalcin acts as a hormone that confers the signal of the osteoblasts to the peripheral tissues that regulate energy metabolism: pancreas, muscle, liver, and white adipose tissue. Osteocalcin favors β-cell proliferation, insulin secretion, insulin sensitivity, and energy expenditure. In addition, insulin signaling in osteoblasts is a positive regulator of osteocalcin production and activity, confirming the existence of a pancreas-bone feedback loop. At the molecular level, two transcription factors, the broadly expressed FoxO1 and the osteoblast-enriched ATF4, act in osteoblasts to regulate energy homeostasis by regulating the activity of osteocalcin. In addition to studies in rodents, a growing body of evidence in the clinical literature has confirmed a favorable link between osteocalcin and energy homeostasis, suggesting that the metabolic functions of the skeleton are retained in humans.
Similar content being viewed by others
References
Yamamoto M, et al. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24(4):702–9.
Janghorbani M, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505.
Schwartz AV, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–8.
Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207.
Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17.
Ferron M, et al. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105(13):5266–70.
Ferron M, et al. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2011;50:568–75.
Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69.
Rached MT, et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120(1):357–68.
Yoshizawa T, et al. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119(9):2807–17.
Kronenberg HM, et al. Principles of endocrinology. In: Kronenberg HM, et al., editors. Williams textbook of endocrinology. Philadelphia: Elsevier; 2008. p. 3–11.
Oury F, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5):796–809.
Ferron M, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308.
Fulzele K, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–19.
Fernandez-Real JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94(1):237–45.
Pittas AG, et al. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94(3):827–32.
Schafer AL, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH Study). J Clin Endocrinol Metab. 2011;96:E1982–9.
Yeap BB, et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol. 2010;163(2):265–72.
Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26(4):677–80.
Mauro LJ, et al. Identification of a hormonally regulated protein tyrosine phosphatase associated with bone and testicular differentiation. J Biol Chem. 1994;269(48):30659–67.
Dacquin R, et al. Knock-in of nuclear localised beta-galactosidase reveals that the tyrosine phosphatase Ptprv is specifically expressed in cells of the bone collar. Dev Dyn. 2004;229(4):826–34.
Maduro MR, et al. Osteotesticular protein tyrosine phosphatase expression in rodent testis. J Urol. 2002;167(5):2282–3.
Wheeler MA, et al. Transcriptional activation of the tyrosine phosphatase gene, OST-PTP, during osteoblast differentiation. J Cell Biochem. 2002;87(4):363–76.
Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–39.
Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19(5):161–6.
Hauschka PV, et al. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047.
Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54(6):1291–7.
Kanazawa I, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22(1):187–94.
Yamauchi M, et al. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 2010;29(6):761–5.
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–49.
Pi M, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3(12):e3858.
Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26(7):1680–3.
Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2011;50:437–43.
Kousteni S. FoxO1: a molecule for all seasons. J Bone Miner Res. 2011;26(5):912–7.
Yang X, Karsenty G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J Biol Chem. 2004;279(45):47109–14.
Yoshikawa Y, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26(9):2012–25.
Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12.
Ducy P, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–54.
Yadav VK, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–89.
Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20.
Hinoi E, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183(7):1235–42.
Cousin W, et al. Cloning of hOST-PTP: the only example of a protein-tyrosine-phosphatase the function of which has been lost between rodent and human. Biochem Biophys Res Commun. 2004;321(1):259–65.
Bacchetta J, et al. The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol Dial Transplant. 2009;24(10):3120–5.
Im JA, et al. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008;396(1–2):66–9.
Kanazawa I, et al. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone. 2011;48(4):720–5.
Kanazawa I, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(1):45–9.
Kindblom JM, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24(5):785–91.
Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes (Lond). 2010;34(5):852–8.
Rochefort GY, et al. Osteocalcin-insulin relationship in obese children: a role for the skeleton in energy metabolism. Clin Endocrinol (Oxf). 2011;75(2):265–70.
Zhou M, et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161(5):723–9.
Goliasch G, et al. Markers of bone metabolism in premature myocardial infarction (</= 40 years of age). Bone. 2011;48(3):622–6.
Winhofer Y, et al. Osteocalcin is related to enhanced insulin secretion in gestational diabetes mellitus. Diabetes Care. 2010;33(1):139–43.
Pollock NK, et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J Clin Endocrinol Metab. 2011;96(7):E1092–9.
Black DM, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, B.C., Kousteni, S. Role of Osteoblasts in Regulation of Energy Metabolism. Clinic Rev Bone Miner Metab 11, 2–10 (2013). https://doi.org/10.1007/s12018-012-9128-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-012-9128-8