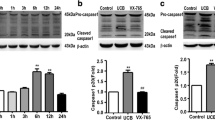
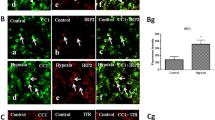
Abstract
Research on the mechanisms of bilirubin-induced neurological dysfunction focuses mainly on neuronal death, astrocyte-mediated events and microglia activation. Although myelin damage by unconjugated bilirubin (UCB) has been documented in neonatal kernicterus cases, the events leading to myelination impairment were never explored. This condition may occur by reduced oligodendrocyte precursor cells (OPC) number, or failure of OPC to differentiate in myelinating oligodendrocytes. We have shown that UCB elicits an inflammatory response, glutamate release and reactive oxygen species (ROS) generation in neurons and glial cells, biomolecules with toxic properties on OPC. Hence, we propose to examine whether UCB determines OPC demise and, if so, which signaling pathways are involved. Our results show that OPC display increased apoptosis and necrosis-like cell death upon UCB exposure, mediated by early signals of endoplasmic reticulum (ER) stress [e.g. upregulation of glucose-regulated protein (GRP)78, inositol-requiring enzyme (IRE)-1α and activation transcription factor (ATF)-6, as well as activation of caspase-2 and c-Jun N-terminal kinase (JNK)], followed by mitochondrial dysfunction (e.g. loss of mitochondria membrane potential and caspase-9 activation). The later calpain activation points to intracellular Ca2+ overload and intervention of both ER and mitochondria. Downstream production of ROS may derive from mitochondria damage and secondary injuries, possibly determining the second cycle of GRP78, IRE-1α, caspase-2 and JNK activation. Moreover, inhibition of caspases, calpains and oxidative stress, by using specific inhibitors, prevented UCB-induced OPC death. UCB did not induce the release of cytokines or glutamate by OPC. These results indicate that UCB by reducing OPC survival, through a cascade of programmed intracellular events triggered by ER stress and mitochondria dysfunction, can compromise myelinogenesis.









Similar content being viewed by others
References
Ahdab-Barmada, M., & Moossy, J. (1984). The neuropathology of kernicterus in the premature neonate: Diagnostic problems. Journal of Neuropathology and Experimental Neurology, 43(1), 45–56.
Benarroch, E. E. (2009). Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology, 72(20), 1779–1785.
Benjamins, J. A., Nedelkoska, L., & George, E. B. (2003). Protection of mature oligodendrocytes by inhibitors of caspases and calpains. Neurochemical Research, 28(1), 143–152.
Bernales, S., Papa, F. R., & Walter, P. (2006). Intracellular signaling by the unfolded protein response. Annual Review of Cell and Developmental Biology, 22, 487–508.
Bogler, O., Wren, D., Barnett, S. C., Land, H., & Noble, M. (1990). Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci USA, 87(16), 6368–6372.
Brites, D. (2011). Bilirubin injury to neurons and glial cells: new players, novel targets, and newer insights. Seminars in Perinatology, 35(3), 114–120.
Brito, M. A., Brites, D., & Butterfield, D. A. (2004). A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Research, 1026(1), 33–43.
Brito, M. A., Lima, S., Fernandes, A., Falcão, A. S., Silva, R. F., Butterfield, D. A., et al. (2008a). Bilirubin injury to neurons: Contribution of oxidative stress and rescue by glycoursodeoxycholic acid. Neurotoxicology, 29(2), 259–269.
Brito, M. A., Rosa, A. I., Falcão, A. S., Fernandes, A., Silva, R. F., Butterfield, D. A., et al. (2008b). Unconjugated bilirubin differentially affects the redox status of neuronal and astroglial cells. Neurobiology of Diseases, 29(1), 30–40.
Brito, M. A., Zurolo, E., Pereira, P., Barroso, C., Aronica, E., & Brites, D. (2012). Cerebellar axon/myelin loss, angiogenic sprouting and neuronal increase of vascular endothelial growth factor in a preterm infant with kernicterus. Journal Child of Neurology, 27(5), 615–624.
Calligaris, R., Bellarosa, C., Foti, R., Roncaglia, P., Giraudi, P., Krmac, H., et al. (2009). A transcriptome analysis identifies molecular effectors of unconjugated bilirubin in human neuroblastoma SH-SY5Y cells. BMC Genomics, 10, 543.
Carragher, N. O., & Frame, M. C. (2002). Calpain: A role in cell transformation and migration. International Journal of Biochemistry and Cell Biology, 34(12), 1539–1543.
Casaccia-Bonnefil, P. (2000). Cell death in the oligodendrocyte lineage: A molecular perspective of life/death decisions in development and disease. Glia, 29(2), 124–135.
Chang, F. Y., Lee, C. C., Huang, C. C., & Hsu, K. S. (2009). Unconjugated bilirubin exposure impairs hippocampal long-term synaptic plasticity. PLoS One, 4(6), e5876.
Chen, H. C., Wang, C. H., Tsan, K. W., & Chen, Y. C. (1971). An electron microscopic and radioautographic study on experimental kernicterus. II. Bilirubin movement within neurons and release of waste products via astroglia. American Journal of Pathology, 64(1), 45–66.
Chen, Y., Balasubramaniyan, V., Peng, J., Hurlock, E. C., Tallquist, M., Li, J., et al. (2007). Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nature Protocols, 2(5), 1044–1051.
Chen, L., Feng, X. C., Lu, F., Xu, X. L., Zhou, G. H., Li, Q. Y., et al. (2011). Effects of camptothecin, etoposide and Ca2+ on caspase-3 activity and myofibrillar disruption of chicken during postmortem ageing. Meat Science, 87(3), 165–174.
Chew, L. J., Coley, W., Cheng, Y., & Gallo, V. (2010). Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. Journal of Neuroscience, 30(33), 11011–11027.
Claireaux, A. E. (1961). Pathology of human kernicterus. In A. Sass-Kortsak (Ed.), Kernicterus: A Report Based on a Symposium Held at the IX International Congress of Paediatrics (pp. 140–149). Toronto: University of Toronto Press.
Deganuto, M., Cesaratto, L., Bellarosa, C., Calligaris, R., Vilotti, S., Renzone, G., et al. (2010). A proteomic approach to the bilirubin-induced toxicity in neuronal cells reveals a protective function of DJ-1 protein. Proteomics, 10(8), 1645–1657.
Deng, W., Rosenberg, P. A., Volpe, J. J., & Jensen, F. E. (2003). Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA, 100(11), 6801–6806.
Deng, W., Pleasure, J., & Pleasure, D. (2008). Progress in periventricular leukomalacia. Archives of Neurology, 65(10), 1291–1295.
Falcão, A. S., Fernandes, A., Brito, M. A., Silva, R. F., & Brites, D. (2005). Bilirubin-induced inflammatory response, glutamate release, and cell death in rat cortical astrocytes are enhanced in younger cells. Neurobiology of Diseases, 20(2), 199–206.
Falcão, A. S., Fernandes, A., Brito, M. A., Silva, R. F., & Brites, D. (2006). Bilirubin-induced immunostimulant effects and toxicity vary with neural cell type and maturation state. Acta Neuropathologica, 112(1), 95–105.
Fernandes, A., & Brites, D. (2009). Contribution of inflammatory processes to nerve cell toxicity by bilirubin and efficacy of potential therapeutic agents. Current Pharmaceutical Design, 15(25), 2915–2926.
Fernandes, A., Silva, R. F., Falcão, A. S., Brito, M. A., & Brites, D. (2004). Cytokine production, glutamate release and cell death in rat cultured astrocytes treated with unconjugated bilirubin and LPS. Journal of Neuroimmunology, 153(1–2), 64–75.
Fernandes, A., Falcão, A. S., Silva, R. F., Brito, M. A., & Brites, D. (2007). MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. European Journal of Neuroscience, 25(4), 1058–1068.
Fernandes, A., Barateiro, A., Falcão, A. S., Silva, S. L., Vaz, A. R., Brito, M. A., et al. (2011). Astrocyte reactivity to unconjugated bilirubin requires TNF-alpha and IL-1beta receptor signaling pathways. Glia, 59(1), 14–25.
Fields, R. D., & Stevens-Graham, B. (2002). New insights into neuron-glia communication. Science, 298(5593), 556–562.
Franco, S., Perrin, B., & Huttenlocher, A. (2004). Isoform specific function of calpain 2 in regulating membrane protrusion. Experimental Cell Research, 299(1), 179–187.
Fu, Y., Sun, W., Shi, Y., Shi, R., & Cheng, J. X. (2009). Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS One, 4(8), e6705.
Galluzzi, L., Blomgren, K., & Kroemer, G. (2009). Mitochondrial membrane permeabilization in neuronal injury. Nature Reviews Neuroscience, 10(7), 481–494.
Gard, A. L., & Pfeiffer, S. E. (1990). Two proliferative stages of the oligodendrocyte lineage (A2B5 + O4- and O4 + GalC-) under different mitogenic control. Neuron, 5(5), 615–625.
Genc, S., Genc, K., Kumral, A., Baskin, H., & Ozkan, H. (2003). Bilirubin is cytotoxic to rat oligodendrocytes in vitro. Brain Research, 985(2), 135–141.
Gkoltsiou, K., Tzoufi, M., Counsell, S., Rutherford, M., & Cowan, F. (2008). Serial brain MRI and ultrasound findings: Relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Human Development, 84(12), 829–838.
Goll, D. E., Thompson, V. F., Li, H., Wei, W., & Cong, J. (2003). The calpain system. Physiological Reviews, 83(3), 731–801.
Gomes, A., Fernandes, E., & Lima, J. L. (2005). Fluorescence probes used for detection of reactive oxygen species. Journal of Biochemical and Biophysical Methods, 65(2–3), 45–80.
Gordo, A. C., Falcão, A. S., Fernandes, A., Brito, M. A., Silva, R. F., & Brites, D. (2006). Unconjugated bilirubin activates and damages microglia. Journal of Neuroscience Research, 84(1), 194–201.
Gu, C., Casaccia-Bonnefil, P., Srinivasan, A., & Chao, M. V. (1999). Oligodendrocyte apoptosis mediated by caspase activation. Journal of Neuroscience, 19(8), 3043–3049.
Gu, H., Chen, X., Gao, G., & Dong, H. (2008). Caspase-2 functions upstream of mitochondria in endoplasmic reticulum stress-induced apoptosis by bortezomib in human myeloma cells. Molecular Cancer Therapeutics, 7(8), 2298–2307.
Gurba, P. E., & Zand, R. (1974). Bilirubin binding to myelin basic protein, histones and its inhibition in vitro of cerebellar protein synthesis. Biochemical and Biophysical Research Communications, 58(4), 1142–1147.
Hansen, T. W. R. (2000). Pioneers in the scientific study of neonatal jaundice and kernicterus. Pediatrics, 106(2), E15.
Hansen, T., Tommarello, S., & Allen, J. (2001). Subcellular localization of bilirubin in rat brain after in vivo i.v. administration of [3H]bilirubin. Pediatric Research, 49(2), 203–207.
Jew, J. Y., & Williams, T. H. (1977). Ultrastructural aspects of bilirubin encephalopathy in cochlear nuclei of the Gunn rat. Journal of Anatomy, 124(Pt 3), 599–614.
Kassmann, C. M., & Nave, K. A. (2008). Oligodendroglial impact on axonal function and survival—a hypothesis. Current Opinion in Neurology, 21(3), 235–241.
Khorchid, A., Fragoso, G., Shore, G., & Almazan, G. (2002). Catecholamine-induced oligodendrocyte cell death in culture is developmentally regulated and involves free radical generation and differential activation of caspase-3. Glia, 40(3), 283–299.
Kim, R., Emi, M., Tanabe, K., & Murakami, S. (2006). Role of the unfolded protein response in cell death. Apoptosis, 11(1), 5–13.
Kotter, M. R., Stadelmann, C., & Hartung, H. P. (2011). Enhancing remyelination in disease—can we wrap it up? Brain, 134(Pt 7), 1882–1900.
Kraskiewicz, H., & FitzGerald, U. (2011). Partial XBP1 knockdown does not affect viability of oligodendrocyte precursor cells exposed to new models of hypoxia and ischemia in vitro. Journal of Neuroscience Research, 89(5), 661–673.
Kulkarni, P., Rajagopalan, K., Yeater, D., & Getzenberg, R. H. (2011). Protein folding and the order/disorder paradox. Journal of Cellular Biochemistry, 112(7), 1949–1952.
Lauer, B. J., & Spector, N. D. (2011). Hyperbilirubinemia in the newborn. Pediatrics in Review, 32(8), 341–349.
Levine, J. M., Reynolds, R., & Fawcett, J. W. (2001). The oligodendrocyte precursor cell in health and disease. Trends in Neurosciences, 24(1), 39–47.
Li, J., & Lee, A. S. (2006). Stress induction of GRP78/BiP and its role in cancer. Current Molecular Medicine, 6(1), 45–54.
Limperopoulos, C. (2010). Advanced neuroimaging techniques: Their role in the development of future fetal and neonatal neuroprotection. Seminars in Perinatology, 34(1), 93–101.
Lin, W., & Popko, B. (2009). Endoplasmic reticulum stress in disorders of myelinating cells. Nature Neuroscience, 12(4), 379–385.
Liu, M. C., Kobeissy, F., Zheng, W., Zhang, Z., Hayes, R. L., & Wang, K. K. (2011). Dual vulnerability of tau to calpains and caspase-3 proteolysis under neurotoxic and neurodegenerative conditions. ASN Neuro, 3(1), e00051.
Lopes, J. P., Oliveira, C. R., & Agostinho, P. (2010). Neurodegeneration in an Abeta-induced model of Alzheimer’s disease: The role of Cdk5. Aging Cell, 9(1), 64–77.
Mancini, M., Machamer, C. E., Roy, S., Nicholson, D. W., Thornberry, N. A., Casciola-Rosen, L. A., et al. (2000). Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. Journal of Cell Biology, 149(3), 603–612.
Martich-Kriss, V., Kollias, S. S., & Ball, W. S., Jr. (1995). MR findings in kernicterus. American Journal of Neuroradiology (AJNR), 16(4 Suppl), 819–821.
Mato, S., Victoria Sánchez-Gómez, M., & Matute, C. (2010). Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia, 58(14), 1739–1747.
McDonagh, A. F., & Assisi, F. (1972). The ready isomerization of bilirubin IX- in aqueous solution. Biochemical Journal, 129(3), 797–800.
McTigue, D. M., & Tripathi, R. B. (2008). The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of Neurochemistry, 107(1), 1–19.
Miller, R. H. (2002). Regulation of oligodendrocyte development in the vertebrate CNS. Progress in Neurobiology, 67(6), 451–467.
Monge, M., Kadiiski, D., Jacque, C. M., & Zalc, B. (1986). Oligodendroglial expression and deposition of four major myelin constituents in the myelin sheath during development. An in vivo study. Developmental Neuroscience, 8(4), 222–235.
Mukhopadhyay, K., Chowdhary, G., Singh, P., Kumar, P., & Narang, A. (2010). Neurodevelopmental outcome of acute bilirubin encephalopathy. Journal of Tropical Pediatrics, 56(5), 333–336.
Murakami, Y., Aizu-Yokota, E., Sonoda, Y., Ohta, S., & Kasahara, T. (2007). Suppression of endoplasmic reticulum stress-induced caspase activation and cell death by the overexpression of Bcl-xL or Bcl-2. Journal of Biochemistry, 141(3), 401–410.
Nave, K. A. (2010). Myelination and support of axonal integrity by glia. Nature, 468(7321), 244–252.
Nishiyama, A., Lin, X. H., Giese, N., Heldin, C. H., & Stallcup, W. B. (1996). Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. Journal of Neuroscience Research, 43(3), 299–314.
Oakes, G. H., & Bend, J. R. (2010). Global changes in gene regulation demonstrate that unconjugated bilirubin is able to upregulate and activate select components of the endoplasmic reticulum stress response pathway. Journal of Biochemical and Molecular Toxicology, 24(2), 73–88.
Özkan, H., Akkoç, N., Aydin, A., Kavukçu, S., Olgun, N., Írken, G., et al. (1995). Relationship between serum unconjugated bilirubin levels and the autofluorescence of white blood cells in neonatal jaundice. Biology of the Neonate, 68(2), 100–103.
Palmela, I., Cardoso, F. L., Bernas, M., Correia, L., Vaz, A. R., Silva, R. F., et al. (2011). Elevated levels of bilirubin and long-term exposure impair human brain microvascular endothelial cell integrity. Current Neurovascular Research, 8(2), 153–169.
Panaretakis, T., Laane, E., Pokrovskaja, K., Bjorklund, A. C., Moustakas, A., Zhivotovsky, B., et al. (2005). Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Molecular Biology of the Cell, 16(8), 3821–3831.
Pfeiffer, S. E., Warrington, A. E., & Bansal, R. (1993). The oligodendrocyte and its many cellular processes. Trends in Cell Biology, 3(6), 191–197.
Pirianov, G., Jesurasa, A., & Mehmet, H. (2006). Developmentally regulated changes in c-Jun N-terminal kinase signalling determine the apoptotic response of oligodendrocyte lineage cells. Cell Death and Differentiation, 13(3), 531–533.
Romisch, K. (2005). Endoplasmic reticulum-associated degradation. Annual Review of Cell and Developmental Biology, 21, 435–456.
Ruiz, A., Matute, C., & Alberdi, E. (2010). Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death and Disease, 1, e54.
Rutkowski, D. T., & Kaufman, R. J. (2004). A trip to the ER: Coping with stress. Trends in Cell Biology, 14(1), 20–28.
Saatman, K. E., Creed, J., & Raghupathi, R. (2010). Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics, 7(1), 31–42.
Sánchez-Gómez, M. V., Alberdi, E., Ibarretxe, G., Torre, I., & Matute, C. (2003). Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. Journal of Neuroscience, 23(29), 9519–9528.
Sánchez-Gómez, M. V., Alberdi, E., Perez-Navarro, E., Alberch, J., & Matute, C. (2011). Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. Journal of Neuroscience, 31(8), 2996–3006.
Shapiro, S. M. (2010). Chronic bilirubin encephalopathy: Diagnosis and outcome. Seminars in Fetal and Neonatal Medicine, 15(3), 157–163.
Silberberg, D. H., & Schutta, H. S. (1967). The effects of unconjugated bilirubin and related pigments on cultures of rat cerebellum. Journal of Neuropathology and Experimental Neurology, 26(4), 572–583.
Silva, R., Mata, L. R., Gulbenkian, S., Brito, M. A., Tiribelli, C., & Brites, D. (1999). Inhibition of glutamate uptake by unconjugated bilirubin in cultured cortical rat astrocytes: Role of concentration and pH. Biochemical and Biophysical Research Communications, 265(1), 67–72.
Silva, R. F., Rodrigues, C. M., & Brites, D. (2001). Bilirubin-induced apoptosis in cultured rat neural cells is aggravated by chenodeoxycholic acid but prevented by ursodeoxycholic acid. Journal of Hepatology, 34(3), 402–408.
Silva, S. L., Vaz, A. R., Barateiro, A., Falcão, A. S., Fernandes, A., Brito, M. A., et al. (2010). Features of bilirubin-induced reactive microglia: From phagocytosis to inflammation. Neurobiology of Diseases, 40(3), 663–675.
Sorimachi, H., Ishiura, S., & Suzuki, K. (1997). Structure and physiological function of calpains. Biochemical Journal, 328(Pt 3), 721–732.
Upton, J. P., Austgen, K., Nishino, M., Coakley, K. M., Hagen, A., Han, D., et al. (2008). Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Molecular and Cellular Biology, 28(12), 3943–3951.
Vaz, A. R., Delgado-Esteban, M., Brito, M. A., Bolaños, J. P., Brites, D., & Almeida, A. (2010). Bilirubin selectively inhibits cytochrome c oxidase activity and induces apoptosis in immature cortical neurons: assessment of the protective effects of glycoursodeoxycholic acid. Journal of Neurochemistry, 112(1), 56–65.
Vaz, A. R., Silva, S. L., Barateiro, A., Falcão, A. S., Fernandes, A., Brito, M. A., et al. (2011a). Selective vulnerability of rat brain regions to unconjugated bilirubin. Molecular and Cellular Neuroscience, 48(1), 82–93.
Vaz, A. R., Silva, S. L., Barateiro, A., Fernandes, A., Falcão, A. S., Brito, M. A., et al. (2011b). Pro-inflammatory cytokines intensify the activation of NO/NOS, JNK1/2 and caspase cascades in immature neurons exposed to elevated levels of unconjugated bilirubin. Experimental Neurology, 229(2), 381–390.
Verma, G., & Datta, M. (2012). The critical role of JNK in the ER-mitochondrial crosstalk during apoptotic cell death. Journal of Cellular Physiology, 227(5), 1791–1795.
Waldbaum, S., & Patel, M. (2010). Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Research, 88(1), 23–45.
Wu, J., & Kaufman, R. J. (2006). From acute ER stress to physiological roles of the unfolded protein response. Cell Death and Differentiation, 13(3), 374–384.
Acknowledgments
This work was supported by the strategic project PEst-OE/SAU/UI4013/2011 and PTDC/SAU-NEU/64385/2006 grants from Fundação para a Ciência e a Tecnologia (FCT), Lisbon, Portugal (to D. B.). A. B. was recipient of a PhD fellowship (SFRH/BD/43885/2008) from FCT. The funding organization had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12017_2012_8187_MOESM1_ESM.tiff
Inhibition of caspases, calpains and oxidative stress prevented unconjugated bilirubin (UCB)-induced oligodendrocyte precursor cell (OPC) death. Rat cortical OPC were exposed to 50 μM UCB in the presence of 100 μM human serum albumin and to specific inhibitors for caspases (Z-VAD-FMK, 1 μM), calpains (calpeptin, 25 μM) or oxidative stress (N-acetylcysteine – NAC, 100 μM). a Caspase-9, caspase-2 and caspase-3 activities with or without Z-VAD-FMK incubation were determined by colorimetric substrate cleavage assay as indicated in Materials and Methods at 4, 8 and 24 h, respectively. Graph bars represent the fold change from vehicle (mean ± SEM), from at least three independent experiments. b Reactive oxygen species (ROS) were determined at 6 h by fluorescence assay with dihydrorhodamine 123 as described in Materials and Methods after incubation in the presence or absence of NAC. Representative images of one experiment and graph bars with fold change from vehicle (mean ± SEM) for ROS production from at least three independent experiments are shown. c Calpain activity was determined at 4 h of incubation using a specific fluorogenic substrate as indicated in Materials and Methods after treatment with or without calpeptin. Representative images of one experiment and graph bars with fold change from vehicle (mean ± SEM) for calpain activation from at least three independent experiments are shown. **P < 0.01 vs. respective vehicle; ## P < 0.01 vs. respective UCB. (TIFF 9060 kb)
12017_2012_8187_MOESM2_ESM.tiff
Unconjugated bilirubin (UCB) induces an increase in ATF-6 and IRE-1α, chaperones usually involved in endoplasmic reticulum stress, in oligodendrocyte precursor cells (OPC). Rat cortical OPC were exposed to 50 μM UCB in the presence of 100 μM human serum albumin for the indicated time periods. a Representative results of ATF-6 and IRE-1α protein expression by Western blot using specific antibodies are shown. b Graph bars represent the fold increase from vehicle (mean ± SEM) obtained for protein band intensity by scanning densitometry standardized with respect to β-actin protein for ATF-6 and IRE-1α, determined as indicated in Materials and Methods, from at least four independent experiments. **P < 0.01 and *P < 0.05 vs. respective vehicle. (TIFF 2855 kb)
Rights and permissions
About this article
Cite this article
Barateiro, A., Vaz, A.R., Silva, S.L. et al. ER Stress, Mitochondrial Dysfunction and Calpain/JNK Activation are Involved in Oligodendrocyte Precursor Cell Death by Unconjugated Bilirubin. Neuromol Med 14, 285–302 (2012). https://doi.org/10.1007/s12017-012-8187-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-012-8187-9