Abstract
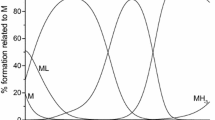
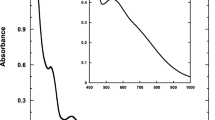
The influence of the oxydiacetate (ODA) and thiodiacetate (TDA) ligands on the physicochemical and biological properties of the oxidovanadium(IV) ternary complexes of the VO(L)(B) type, where L denotes ODA or TDA and B denotes 2,2′-bipyridine (bipy) or 1,10-phenanthroline (phen), has been investigated. The stability of the complexes in aqueous solutions, assessed based on the potentiometric titration method, increases in the following direction: VO(TDA)(bipy) < VO(ODA)(bipy) < VO(TDA)(phen) < VO(ODA)(phen). Furthermore, the influence of the TDA and ODA ligands on the antioxidant activity of the oxidovanadium(IV) complexes toward superoxide free radical (O2 •−), 2,2′-azinobis(3-ethylbenzothiazoline-6 sulfonic acid) cation radical (ABTS+•) and 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) has been examined and discussed. The reactivity of the complexes toward O2 •− increases in the following direction: VO(TDA)(phen) < VO(TDA)(bipy) ≈ VO(ODA)(bipy) < VO(ODA)(phen). The antioxidant activity against ABTS+• and DPPH• free radicals is higher for phen complexes, whereas the thiodiacetate complexes are more reactive than are the corresponding oxydiacetate ones. Finally, herein, the cytoprotective activity of the complexes against the oxidative damage generated exogenously by hydrogen peroxide in the hippocampal neuronal HT22 cell line (the MTT and LDH tests) is reported. In a low concentration (1 μM), the cytoprotective action of thiodiacetate complexes is much higher than that of the corresponding oxydiacetate complexes. However, in the higher concentration range (10 and 100 μM), the antioxidant activity of the complexes is overcompensated by their cytotoxic effect.








Similar content being viewed by others
References
Rehder D (1991) Biologische chemie des vanadiums: biomimetische vanadium-verbindungen als katalysatoren und antidiabetika. Angew Chem, Int Ed Engl 30:148–167
Pessoa JC, Etcheverry S, Gambino D (2015) Vanadium compounds in medicine. Coord Chem Rev 301-302:24–48
Pessoa JC, Garribba E, Santos MF, Santos-Silva T (2015) Vanadium and proteins: Uptake, transport, structure, activity and function. Coord Chem Rev 301-302:49–86
Kioseoglou E, Petanidis S, Gabriel C, Salifoglou A (2015) The chemistry and biology of vanadium compounds in cancer therapeutics. Coord Chem Rev 301-302:87–105
Rehder D (2013) The future of/for vanadium. Dalton Trans 42:11749–11761
Pessoa JC, Tomaz I (2010) Transport of therapeutic vanadium and ruthenium complexes by blood plasma components. Curr Med Chem 17:3701–3738
Srivastava AK, Mehdi MZ (2005) Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabetic Med 22:2–13
Marzban L, McNeill JH (2003) Insulin-like actions of vanadium: potential as a therapeutic agent. J Trace Elem Exp Med 16:253–267
Thompson KH, Lichter J, LeBel C, Scaife MC, McNeill JH, Orvig C (2009) Vanadium treatment of type 2 diabetes: a view to the future. J Inorg Biochem 103:554–558
McNeill JH, Yuen VG, Hoveyda HR, Orvig C (1992) Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J Med Chem 35:1489–1491
Levina A, Lay PA (2011) Metal-based anti-diabetic drugs: advances and challenges. Dalton Trans 40:11675–11686
Thompson KH, Orvig C (2006) Vanadium in diabetes: 100 years from Phase 0 to Phase I. J Inorg Biochem 100:1925–1935
Rivadeneira J, Di Virgilio AL, Barrio DA, Muglia CI, Bruzzone L, Etcheverry SB (2010) Cytotoxicity of a vanadyl(IV) complex with a multidentate oxygen donor in osteoblast cell lines in culture. Med Chem 6:9–23
Rivadeneira J, Barrio DA, Etcheverry SB, Baran EJ (2007) Spectroscopic characterization of a VO2+ complex of oxodiacetic acid and its bioactivity on osteoblast-like cells in culture. Biol Trace Elem Res 118:159–166
León IE, Etcheverry SB, Parajón-Costa BS, Baran EJ (2012) Spectroscopic characterization of an oxovanadium(IV) complex of oxodiacetic acid and o-Phenanthroline. bioactivity on osteoblast-like cells in culture. Biol Trace Elem Res 147:403–407
Wyrzykowski D, Inkielewicz-Stępniak I, Czupryniak J, Jacewicz D, Ossowski T, Woźniak M, Chmurzyński L (2013) Electrochemical and biological studies on reactivity of [VO(oda)(H2O)2], [Co(oda)(H2O)2]·H2O, and [Ni(oda)(H2O)3]·1.5H2O towards superoxide free radicals. Z Anorg Allg Chem 639:1795–1799
Wyrzykowski D, Inkielewicz-Stępniak I, Pranczk J, Żamojć K, Zięba P, Tesmar A, Jacewicz D, Ossowski T, Chmurzyński L (2015) Physicochemical properties of ternary oxovanadium (IV) complexes with oxydiacetate and 1, 10-phenanthroline or 2,2′-bipyridine. cytoprotective activity in hippocampal neuronal HT22 cells. Biometals 28:307–320
Álvarez L, Grirrane A, Moyano R, Álvarez E, Pastor A, Galindo A (2010) Comparison of the coordination capabilities of thiodiacetate and oxydiacetate ligands through the X-ray characterization and DFT studies of [V(O)(tda)(phen)]∙4H2O and [V(O)(oda)(phen)]∙1.5H2O. Polyhedron 29:3028–3035
Pranczk J, Wyrzykowski D, Jacewicz D, Sikorski A, Tesmar A, Chmurzyński L (2015) Structural, physico-chemical and antioxidant characteristics of 2,2′-bipyridyl(iminodiacetato)oxidovanadium(IV)dihydrate. Polyhedron 100:74–81
Pranczk J, Jacewicz D, Wyrzykowski D, Wojtczak A, Tesmar A, Chmurzyński L (2015) Crystal structure, antioxidant properties and characteristics in aqueous solutions of the oxidovanadium(IV) complex [VO(IDA) phen]·2H2O. Eur J Inorg Chem 2015:3343–3349
Ramsis H, Perez-Ruiz E, Roger J, Delarbre JL, Maury L (1996) Vibrational study of thiodiglycolic acid and potassium thiodiglycolate salts. J Raman Spectr 27:637–644
Banik B, Somyajit K, Nagaraju G, Chakravarty AR (2014) Oxovanadium(IV) complexes of curcumin for cellular imaging and mitochondria targeted photocytotoxicity. Dalton Trans 43:13358–13369
Wyrzykowski D, Tesmar A, Jacewicz D, Pranczk J, Chmurzyński L (2014) Zinc(II) complexation by some biologically relevant pH buffers. J Mol Recognit 27:722–726
Brandariz I, Barriada J, Vilarino T, de Vicente MS (2004) Comparison of several calibration procedures for glass electrodes in proton concentration. Monatsh Chem 135:1475–1488
Chmurzyński L (1996) Studies on correlations of acid-base properties of substituted pyridine N-oxides in solutions. Part 2. correlations of the pKa values in non-aqueous media. Anal Chim Acta 326:267–274
Chmurzyński L, Nesterowicz M, Wawrzyniak G, Kaczmarczyk E, Warnke Z (1996) A potentiometric study on proton-transfer equilibria and cationic conjugation in pyridine N-oxides systems in acetone and methanol. Aust J Chem 49:931–942
Wawrzynów A, Chmurzyński L (1998) A comparison of acid-base properties of substituted pyridines and their N-oxides in propylene carbonate. J Chem Thermodyn 30:713–722
Gans P, Sabatini A, Vacca A (1996) Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43:1739–1753
Sillen LG, Martel AE (1966) Stability constants of metal-ion complexes, the chemical Society. Great Britain, London
Henry RP, Mitchell PCH, Prue JE (1973) Hydrolysis of the oxovanadium(IV) ion and the stability of its complexes with the 1,2-dihydroxybenzenato(2–) ion. J Chem Soc Dalton Trans 1156–1159
Wyrzykowski D, Anusiewicz I, Pilarski B, Jacewicz D, Chmurzyński L (2013) Investigations of coordinating properties of oxydiacetate and thiodiacetate anions towards Zn2+ ions in solutions. Inorg Chim Acta 405:163–168
Alderighi L, Gans P, Ienco A, Peters D, Sabatini A, Vacca A (1999) Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord Chem Rev 184:311–318
Tesmar A, Inkielewicz-Stępniak I, Sikorski A, Wyrzykowski D, Jacewicz D, Zięba P, Pranczk J, Ossowski T, Chmurzyński L (2015) Structure, physicochemical and biological properties of new complex salt of aqua-(nitrilotriacetato-N,O,O′,O″)-oxidovanadium(IV) anion with 1,10-phenanthrolinium cation. J Inorg Biochem 152:53–61
Barszcz B (2005) Coordination properties of didentate N,O heterocyclic alcohols and aldehydes towards Cu(II), Co(II), Zn(II) and Cd(II) ions in the solid state and aqueous solution. Coord Chem Rev 249:2259–2276
Wyrzykowski D, Pranczk J, Jacewicz D, Tesmar A, Pilarski B, Chmurzyński L (2014) Investigations of ternary complexes of Co(II) and Ni(II) with oxydiacetate anion and 1,10-phenanthroline or 2,2′-bipyridine in solutions. Cent Eur J Chem 12:107–114
Galloni P, Conte V, Floris B (2015) A journey into electrochemistry of vanadium compounds. Coord Chem Rev 301–302:240–299
León IE, Butenko N, Virgilio ALD, Muglia CI, Baran EJ, Cavaco I, Etcheverry SB (2014) Vanadium and cancer treatment: Antitumoral mechanisms of three oxidovanadium(IV) complexes on a human osteosarcoma cell line. J Inorg Biochem 134:106–117
Yodoshi M, Odoko M, Okabe N (2007) Structures and DNA-binding and cleavage properties of ternary copper(II) complexes of glycine with phenanthroline, bipyridine, and bipyridylamine. Chem Pharm Bull 55:853–860
Chakravarty AR (2006) Photocleavage of DNA by copper(II) complexes. J Chem Sci 118:443–453
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2:219–236
Jacewicz D, Siedlecka-Kroplewska K, Pranczk J, Wyrzykowski D, Woźniak M, Chmurzyński L (2014) Cis-[Cr (C2O4)(pm)(OH2)2]+ coordination ion as a specific sensing ion for H2O2 detection in HT22 cells. Molecules 19:8533–8543
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Jacewicz D, Dąbrowska A, Wyrzykowski D, Pranczk J, Woźniak M, Kubasik-Juraniec J, Knap N, Siedlecka K, Neuwelt AJ, Chmurzyński L (2010) Novel biosensor for evaluation of apoptotic or necrotic effects of nitrogen dioxide during acute pancreatitis in rat. Sensors 10:280–291
Acknowledgments
This research was financially supported by the National Science Centre on the basis of decision number DEC-2012/07/B/ST5/00753.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pranczk, J., Tesmar, A., Wyrzykowski, D. et al. Influence of Primary Ligands (ODA, TDA) on Physicochemical and Biological Properties of Oxidovanadium (IV) Complexes with Bipy and Phen as Auxiliary Ligands. Biol Trace Elem Res 174, 251–258 (2016). https://doi.org/10.1007/s12011-016-0687-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0687-2