Abstract
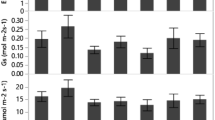
Acer buergerianum Miq. (Trident maple) is a native species of China with a large distribution, but exist in small population. Water and light are two important factors limiting plant growth and are crucial in the framework of forest regeneration. However, there is no consensus on how shade interacts with drought. Four hypotheses in the recent literature variously predict that shade will have a stronger, weaker or equal impact on seedlings under drought stress. This study investigated the interactive responses of A. buergerianum to light and water focusing on seedling growth, leaf morphology and biomass partitioning by performing a growth experiment in pots with different water supply regimes [15, 35, 55, 75, 95 % of field capacity (FC)] combined with two light regimes (10 and 66 % of full sunlight). After 123 days treatment, the results showed that shade greatly reduced growth and biomass, in contrast enhancing the amount of chlorophyll, the amount of water in the leaves, and the specific leaf area. Drought reduced growth, biomass, and the bulk of the leaves. Most leaf traits and biomass characteristics had strong interactions in their responses to light and water treatments. Allometric analysis revealed that water and light had no effects on root to shoot ratios, main root to lateral root ratios, and root mass ratios. Shade alleviated the negative impact of drought. A. buergerianum successfully adapted to the various light and water conditions. We recommend a water supply above 15 % FC to keep the seedlings vigorous, under both sunlight conditions.





Similar content being viewed by others
References
Achten WMJ, Maes WH, Reubens B, Mathijs E, Singh VP, Verchot L, Muys B (2010) Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass and Bioenergy 34(5):667–676
Adams W, Demmig-Adams B, Winter K, Schreiber U (1990) The ratio of variable to maximum chlorophyll fluorescence from photosystem II, measured in leaves at ambient temperature and at 77 K, as an indicator of the photon yield of photosynthesis. Planta 180:166–174
Aranda I, Castro L, Pardos M, Gil L, Pardos J (2005) Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (L.) seedlings. For Ecol Manage 210(1–3):117–129
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Zeigler H (eds) Encyclopedia of Plant Physiology, New Series, Springer, Berlin, 12A: pp 57–107
Du N, Guo WH, Zhang XR, Wang RQ (2010) Morphological and physiological responses of Vitex negundo L. var. heterophylla (Franch.) rehd. to drought stress. Acta Physiol Plant 32(5):839–848
Falster DS, Warton DI, Wright IJ (2006) User’s guide to SMATR: standardized major axis tests and routines, version 2.0. http://wwwbiomqeduau/ecology/SMATR/SMATR_users_guidepdf
Grime JP, Thompson K, Hunt R, Hodgson JG, Cornelissen JHC, Rorison IH, Hendry GAF, Ashenden TW, Askew AP, Band SR, Booth RE, Bossard CC, Campbell BD, Cooper JEL, Davison AW, Gupta PL, Hall W, Hand DW, Hannah MA, Hillier SH, Hodkinson DJ, Jalili A, Liu Z, Mackey JML, Matthews N, Mowforth MA, Neal AM, Reader RJ, Reiling K, Ross-Fraser W, Spencer RE, Sutton F, Tasker DE, Thorpe PC, Whitehouse J (1997) Integrated screening validates primary axes of specialization in plants. Oikos 79:259–281
Guo W, Li B, Zhang X, Wang R (2007) Architectural plasticity and growth responses of Hippophae rhamnoides and Caragana intermedia seedlings to simulated water stress. J Arid Environ 69(3):385–399
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ 25(8):1021–1030
Holmgren M (2000) Combined effects of shade and drought on tulip poplar seedlings: trade-off in tolerance or facilitation? Oikos 90(1):67–78
Holmgren M, Scheffer M, Huston MA (1997) The interplay of facilitation and competition in plant communities. Ecology 78:1966–1975
Humbert L, Gagnon D, Kneeshaw D, Messier C (2007) A shade tolerance index for common understory species of northeastern North America. Ecol Indic 7(1):195–207
Jason JG, Thomas GR, Pharr DM (2004) Photosynthesis, chlorophyll fluorescence, and carbohydrate content of Illicium taxa grown under varied irradiance. J Am Soc Hort Sci 129(1):46–53
Jiménez MD, Pardos M, Puértolas J, Kleczkowski LA, Parados JA (2009) Deep shade alters the acclimation response to moderate water stress in Quercus suber L. Forestry 82(3):285–298
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23:187–261
Lei TT, Lechowicz MJ (1998) Diverse responses of maple saplings to forest light regimes. Ann Bot 82(1):9–19
Lei TT, Tabuchi R, Kitao M, Koike T (1996) Functional relationship between chlorophyll content and leaf reflectance, and light capturing efficiency of Japanese forest species. Physiol Plant 96:411–418
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Navas M-L, Garnier E (2002) Plasticity of whole plant and leaf traits in Rubia peregrina in response to light, nutrient and water availability. Acta Oecologica 23:375–383
Nobel PS (1984) Productivity of Agave deserti: measurement by dry weight and monthly prediction using physiological responses to environmental parameters. Oecologia 64(1):1–7
Nobel PS (1999) Physicochemical and environmental plant physiology, 2nd edn. Academic Press, San Diego
Nobel PS, Hartsoek TL (1986) Temperature, water, and PAR influences on predicted and measured productivity of Agave deserti at various elevations. Oecologia, 181–185
Noda H, Muraoka H, Washitani I (2004) Morphological and physiological acclimation responses to contrasting light and water regimes in Primula sieboldii. Ecol Res 19:331–340
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Quero JL, Villar R, Marañón T, Zamora R (2006) Interactions of drought and shade effects on seedlings of four Quercus species: physiological and structural leaf responses. New Phytol 170(4):819–833
Rozendaal DMA, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct Ecol 20(2):207–216
Sack L, Grubb P (2002) The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 131(2):175–185
Sack L, Grubb PJ, Marañón T (2003) The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecol 168(1):139–163
Smith T, Humston M (1989) A theory of the spatial and temporal dynamics of plant communities. Vegetatio 83:49–69
Wang ML, Jiang YS, Wei JQ, Wei X, Qi XX, Jiang SY, Wang ZM (2008) Effects of irradiance on growth, photosynthetic characteristics, and artemisinin content of Artemisia annua L. Photosynthetica 46(1):17–20
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81(2):259–291
Xu F, Guo W, Xu W, Wei Y, Wang R (2009) Leaf morphology correlates with water and light availability: what consequences for simple and compound leaves? Prog Nat Sci 19(12):1789–1798
Xu F, Guo WH, Xu WH, Wang RQ (2008) Habitat effects on leaf morphological plasticity in Quercus Acutissima. Acta Biol Cracov Bot 50(2):19–26
Yang Y, Han C, Liu Q, Lin B, Wang J (2008) Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol Plant 30(4):433–440
Zhang X, Liu J, Welham C, Liu C, Li D, Chen L, Wang R (2006) The effects of clonal integration on morphological plasticity and placement of daughter ramets in black locust (Robinia pseudoacacia). Flora 201(7):547–554
Acknowledgments
We express our deep gratitude to Yinghua Wei, Chong Fang, Huan He, Yifu Yuan, Chengdong Wang, Wenjuan Ding, Penglin Lu and Hua Chen for their help during the experiments. Thanks to Dr. Jian Liu for his valuable comments and suggestions on the submission, to Dr. Roberta Greenwood of Shandong University for linguistic advice. We also thank two anonymous reviewers and the editor for their insightful and helpful comments. The research was supported by National Science Foundation of China (No.30970166; 31270374), Ministry of Education on Doctorial Discipline (No.20090131110066) and Independent Inovation Project of Shandong University (No.2010JC004; 2011DX008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Franklin.
Rights and permissions
About this article
Cite this article
Guo, X., Guo, W., Luo, Y. et al. Morphological and biomass characteristic acclimation of trident maple (Acer buergerianum Miq.) in response to light and water stress. Acta Physiol Plant 35, 1149–1159 (2013). https://doi.org/10.1007/s11738-012-1154-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1154-0