Abstract
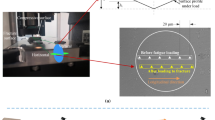
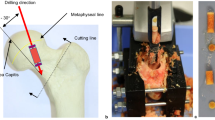
An analytical centrifugation technique (LUMiFuge) for mechanical testing is introduced. Measurements on calibrated springs with LUMiFuge and BOSE LM1-ElectroForce TestBench delivered similar values for E-moduli and spring constants. Native cylindrical scaffolds from cancellous femoral bone were successfully tested using this new technique. The E-moduli obtained for scaffolds with trabecular orientation parallel to the cylindrical axis (longitudinal trabeculae) were significantly higher than that of scaffolds with trabeculae perpendicular to the cylindrical axis (lateral trabeculae). The mean value of elastic modulus increased in dependence on the number of stress rounds from 37.3 ± 19.1 to 63.0 ± 24.2 MPa for samples with longitudinal trabeculae and from 16.0 ± 8.1 to 33.3 ± 13.8 MPa for samples with lateral trabeculae, respectively. We assume that due to the mechanical stress a not completely reversible structural displacement of trabeculae occurs, which results in more compact trabecular arrangement and increase of elastic modulus. The new analytical centrifugation technique offers advantages for characterization of mechanical properties of small bone samples. The method is relatively simple, allows simultaneous testing of several samples, non-destructive testing by application of low loads and can be used to follow the influence of repetitive stress on deformation and recovery of the bone.



Similar content being viewed by others

References
Jerosch J (1990) Knochenbanken in der BRD. Ergebnisse einer Befragung. Unfallchirurg 93:334–338
von Garrel T, Gotzen L (1998) Allogeneic bone transplantation and bone banking. Unfallchirurg 101(9):713–727
Bundesinstitutes für Arzneimittel und Medizinprodukte (1996) Bekanntmachung zu allogenen Gewebetransplantaten. Bundesanzeiger 75:4670
Wissenschaftlicher Beirat der Bundesärztekammer (2001) Richtlinien zum Führen einer Knochenbank. Deutsches Ärzteblatt 98(15):A1011–A1016
Pelker RR, Friedlaender GE, Markham TC (1983) Biomechanical properties of bone allografts. Clin Orthop Relat Res 174:54–57
Kearney JN, Bojar R, Holland KT (1993) Ethylene oxide sterilisation of allogenic bone implants. Clin Mater 12(3):129–135
von Garrel T, Knaepler H, Gürtler L (1997) Evaluation of the inactivation of HIV-1 in human femoral heads by heat treatment to 80 °C (Lobator SD-1. Unfallchirurg 100(5):375–381
Pruss A, Baumann B, Seibold M, et al. (2001) Validation of the sterilization procedure of allogeneic avital bone transplants using peracetic acid-ethanol. Biologicals 29(2):59–66
Sychterz CJ, Orishimo KF, Engh CA (2004) Sterilization and polyethylene wear: clinical studies to support laboratory data. J Bone Joint Surg Am 86(5):1017–1022
Grieb TA, Forng R-Y, Stafford RE, et al. (2005) Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials 26(14):2033–2042
Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W (2005) Irradiation as a safety procedure in tissue banking. Cell Tissue Bank 6(3):201–219
Nguyen H, Morgan DAF, Forwood MR (2007) Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank 8(2):93–105
Diehl P, Schauwecker J, Mittelmeier W, Schmitt M (2008) High hydrostatic pressure, a novel approach in orthopedic surgical oncology to disinfect bone, tendons and cartilage. Anticancer Res 28(6B):3877–3883
Wallace S, Gellin R (2010) Clinical evaluation of freeze-dried cancellous block allografts for ridge augmentation and implant placement in the maxilla. Implant Dent 19(4):272–279
Aspenberg P, Johnsson E, Thorngren K-G (1990) Dose dependent reduction of bone inductive properties by ethylene oxide. J Bone Joint Surg (Br.) 72(6):1036–1037
Anderson MJ, Keyak JH, Skinner HB (1992) Compressive mechanical properties of human cancellous bone after gamma irradiation. J Bone Joint Surg Am 74(5):747–752
Cornu O, Banse X, Docquier PL, Luyckx S, Delloye C (2000) Effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. J Orthop Res 18(3):426–431
Dunsmuir RA, Gallacher G (2003) Microwave sterilization of femoral head allograft. J Clin Microbiol 41(10):4755–4757
Endres S, Kratz M, Heinz M, et al. (2005) Biocompatibility testing of different sterilised or disinfected allogenous bone grafs in comparison to the gold standard of autologous bone grafts—an in vitro analysis of immunomodulation. Z Orthop Ihre Grenzgeb 143(6):660–668
Akkus O, Belaney RM, Das P (2005) Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res 23(4):838–845
An YH, Alvi FI, Kang Q, et al. (2005) Effects of sterilization on implant mechanical property and biocompatibility. Int J Artif Organs 28(11):1126–1137
Bauer J, Liu RW, Kean TJ, Dennis JE, Petersilge W, Gilmore A (2011) A comparison of five treatment protocols for contaminated bone grafts in reference to sterility and cell viability. J Bone Joint Surg Am 93(5):439–444
Knaepler H, von Garrel T, Gotzen L (1994) Untersuchungen zur Desinfektion und Sterilisation allogener Knochentransplantate. Unfallchirurg (Hefte) 235:1–101
Pruss A, Seibold M, Benedix F, et al. (2003) Validation of the “Marburg bone bank system” for thermodisinfection of allogenic femoral head transplants using selected bacteria, fungi, and spores. Biologicals 31(4):287–294
Hallfeldt KK, Stutzle H, Puhlmann M, Kessler S, Shweiberer L (1995) Sterilization of partially deminerallized bone matrix: the effects of different sterilization techniques on osteogenic properties. J Surg Res 59(5):614–620
Currey JD, Foreman J, Laketić I, et al. (1997) Effects of ionizing radiation on the mechanical properties of human bone. Journal of orthopedic. Research 15(1):111–117
Herr G, Schmid U, Holz G, Reutter K, Schnettler R (1997) Einfluss verschiedener Desinfektions- und Sterilisationsverfahren auf die biologische Aktivitä̈t und Struktur von Knochengewebe. In: R. Schnettler, E. Markgraf (eds) Knochenersatzmaterialien und Wachstumsfaktoren, Georg Thieme Verlag, Stuttgart, pp. 84–87
Rauh J, Despang F, Baas J, Liebers C, Pruss A, Gelinsky M, Günther, K-P, Stiehler M (2014) Comparative Biomechanical and Microstructural Analysis of Native versus Peracetic Acid-Ethanol Treated Cancellous Bone Graft. BioMed Research International V Art. ID 784702
Weaver JK (1966) The microscopic hardness of bone. J Bone Joint Surg Am 48(2):273–288
Ryan JC, Williams JL (1989) Tensile testing of rodlike trabeculae excised from bovine femoral bone. J Biomech 22(4):351–355
Hayes WC, Piazza SJ, Zysset PK (1991) Biomechanics of fracture risk prediction of the hip and spine by quantitative computed tomography. Radiol Clin N Am 29(1):1–18
Rho JY, Ashman RB, Turner CH (1993) Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech 26(2):111–119
Keaveny TM, Wachtel EF, Ford CM, Hayes WC (1994) Differences between the tensile and compressive strengths of bovine tibial trabecular bone depend on modulus. J Biomech 27(9):1137–1146
Zysset PK, Guo XE, Hoffner CE, Moore KE, Goldstein SA (1999) Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech 32(10):1005–1012
Acknowledgments
This study was financially supported by Federal Ministry of Economics and Technology of Germany (BMWi) KF 2354408AJ2. We acknowledge the technical support by Mr. Frank Schweiger, Dr. Matthias Deumer and Jan.-Erik Ode. The LUMiFuge was provided by Telos GmbH, Marburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bäumler, H., Hamberger, L., Zaslansky, P. et al. Non-Destructive Mechanical Testing of Allograft Bone-Implants by Analytic Centrifugation. Exp Mech 56, 1653–1660 (2016). https://doi.org/10.1007/s11340-016-0201-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-016-0201-y