Abstract
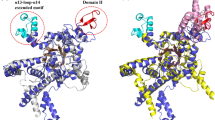
Isocitrate lyase, encoded by the aceA gene, plays an important role in the ability of Pseudomonas aeruginosa to grow on fatty acids, acetate, acyclic terpenes, and amino acids. Phylogenetic analysis indicated that the ICL superfamily is divided in two families: the ICL family, which includes five subfamilies, and the 2-methylisocitrate lyase (MICL) family. ICL from P. aeruginosa (ICL-Pa) was identified in a different ICL node (subfamily 3) than other Pseudomonas ICL enzymes (grouped in subfamily 1). Analysis also showed that psychrophilic bacteria are mainly grouped in ICL subfamily 3, whose ICL proteins contain the highly conserved catalytic pattern QIENQVSDEKQCGHQD. We performed site-directed mutagenesis, enzymatic activity, and structure modeling of conserved residues in mutated ICLs by using ICL-Pa as a model. Our results indicated that the N214 residue is essential for catalytic function, while mutating the Q211, E219, and Q221 residues impairs its catalytic and thermostability properties. Our findings suggest that conserved residues in the subfamily 3 signature of ICL-Pa play important roles in catalysis and thermostability and are likely associated with the catalytic loop structural conformation.





Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bentrup KH, Miczak A, Swenson DL, Russell DG (1999) Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J Bacteriol 181:7161–7167
Britton K, Langridge S, Baker PJ, Weeradechapon K, Sedelnikova SE, De Lucas JR, Rice DW, Turner G (2000) The crystal structure and active site location of isocitrate lyase from the fungus Aspergillus nidulans. Structure 8:349–362
Brock M, Darley D, Textor S, Buckel W (2001) 2-Methylisocitrate lyases from the bacterium Escherichia coli and the filamentous fungus Aspergillus nidulans: characterization and comparison of both enzymes. Eur J Biochem 268:3577–3586
Campos-Garcia J (2010) Metabolism of acyclic terpenes by Pseudomonas. In Pseudomonas, pp 235–253. Edited by Ramos, J. L. and Filloux, A. Springer Netherlands
Chavez-Aviles M, Diaz-Perez AL, Reyes-de la Cruz H, Campos-Garcia J (2009) The Pseudomonas aeruginosa liuE gene encodes the 3-hydroxy-3-methylglutaryl coenzyme A lyase, involved in leucine and acyclic terpene catabolism. FEMS Microbiol Lett 296:117–123
Diaz-Perez AL, Roman-Doval C, Diaz-Perez C, Cervantes C, Sosa-Aguirre CR, Lopez-Meza JE, Campos-Garcia J (2007) Identification of the aceA gene encoding isocitrate lyase required for the growth of Pseudomonas aeruginosa on acetate, acyclic terpenes and leucine. FEMS Microbiol Lett 269:309–316
Diehl P, McFadden BA (1994) The importance of four histidine residues in isocitrate lyase from Escherichia coli. J Bacteriol 176:927–931
Dunn MF, Ramirez-Trujillo JA, Hernandez-Lucas I (2009) Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 155:3166–3175
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113
Fiser A, Sali A (2003) Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol 374:461–491
Gainey LD, Connerton IF, Lewis EH, Turner G, Ballance DJ (1992) Characterization of the glyoxysomal isocitrate lyase genes of Aspergillus nidulans (acuD) and Neurospora crassa (acu-3). Curr Genet 21:43–47
Hagins JM, Locy R, Silo-Suh L (2009) Isocitrate lyase supplies precursors for hydrogen cyanide production in a cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol 191:6335–6339
Higgins DG, Sharp PM (1988) CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237–244
Kondrashov FA, Koonin EV, Morgunov IG, Finogenova TV, Kondrashova MN (2006) Evolution of glyoxylate cycle enzymes in Metazoa: evidence of multiple horizontal transfer events and pseudogene formation. Biology Direct 1:31
Kretzschmar U, Ruckert A, Jeoung JH, Gorisch H (2002) Malate:quinone oxidoreductase is essential for growth on ethanol or acetate in Pseudomonas aeruginosa. Microbiology 148:3839–3847
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary gnetics analysis and sequence alignment. Brief Bioinform 5:150–163
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291
Müller S, Fleck CB, Wilson D, Hummert C, Hube B, Brock M (2011) Gene acquisition, duplication and metabolic specification: the evolution of fungal methylisocitrate lyases. Environ Microbiol 13:1534–1548
Muñoz-Elias EJ, McKinney JD (2005) Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11:638–644
Narayanan B, Niu W, Joosten HJ, Li Z, Kuipers RK, Schaap PJ, Dunaway-Mariano D, Herzberg O (2009) Structure and function of 2,3-dimethylmalate lyase, a PEP mutase/isocitrate lyase superfamily member. J Mol Biol 386:486–503
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harb Lab Press, New York
Sato Y, Watanabe S, Yamaoka N, Takada Y (2008) Gene cloning of cold-adapted isocitrate lyase from a psychrophilic bacterium, Colwellia psychrerythraea, and analysis of amino acid residues involved in cold adaptation of this enzyme. Extremophiles 12:107–117
Watanabe S, Takada Y (2004) Amino acid residues involved in cold adaptation of isocitrate lyase from a psychrophilic bacterium, Colwellia maris. Microbiology 150:3393–3403
Acknowledgments
This research was funded by CONACYT (106567) and CIC/UMSNH 2.14 grants. We thank C. Cervantes by his comments in the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Campos-Garcia, J., Diaz-Perez, C. & Diaz-Perez, A.L. Residues Asn214, Gln211, Glu219 and Gln221 contained in the subfamily 3 catalytic signature of the isocitrate lyase from Pseudomonas aeruginosa are involved in its catalytic and thermal properties. World J Microbiol Biotechnol 29, 991–999 (2013). https://doi.org/10.1007/s11274-013-1258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1258-8