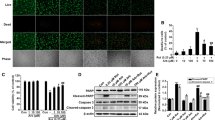
Abstract
Curcumin (CUR), a dietary polyphenol has diverse pharmacologic effects, but is limited by poor bioavailability. This is probably due to decreased solubility, cellular uptake and stability. In order to enhance its solubility and bioavailability, we synthesized the CUR bioconjugate curcumin monoglucoside (CMG) and tested its bioavailability, neuroprotective and anti-apoptotic propensity against rotenone (ROT) induced toxicity in N27 dopaminergic neuronal cells and Drosophila models. Our results elucidate that CMG showed improved bioavailability than CUR in N27 cells. Pre-treatment with CMG protected against ROT neurotoxicity and exerted antioxidant effects by replenishing cellular glutathione levels and significantly decreasing reactive species. CMG pre-treatment also restored mitochondrial complex I and IV activities inhibited by ROT. ROT-induced nuclear damage was also restored by CMG as confirmed by comet assay. CMG induced anti-apoptotic effects was substantiated by decreased phosporylation of JNK3 and c-jun, which in turn decreased the cleavage of pro-caspase 3. Q-PCR analysis of redox genes showed up-regulation of NOS2 and down-regulation of NQO1 upon ROT exposure and this was attenuated by CMG pre-treatment. Studies in the Drosophila ROT model revealed that, CMG administration showed better survival rate and locomotor activity, improved antioxidant activity and dopamine content than ROT treated group and was comparable with the CUR group. Based on these data, we surmise that CMG has improved bioavailability and offered neuroprotection comparable with CUR, against ROT-induced toxicity both in dopaminergic neuronal cell line and Drosophila models, with therapeutic implications for PD.









Similar content being viewed by others
Abbreviations
- CMG:
-
Curcumin monoglucoside
- ROS:
-
Reactive oxygen species
- GSH:
-
Glutathione (reduced)
- CI:
-
Mitochondrial complex I
- CIV:
-
Mitochondrial complex IV
- PD:
-
Parkinson’s disease
- JNK:
-
c-Jun N-terminal kinase.
References
Mercado G, Valdes P, Hetz C (2013) An ERcentric view of Parkinson’s disease. Trends Mol Med 19:165–175
Anand P, Thomas SG, Kunnumakkara AB et al (2008) Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem Pharmacol 76:1590–1611
Shishodia S, Misra K, Aggarwal B (2008) Turmeric as cure-cumin: promises, problems, and solutions. In: Dietary modulation of cell signaling pathways. Taylor & Francis, Inc., p 91
Lim GP, Chu T, Yang F et al (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21:8370–8377
Thiyagarajan M, Sharma SS (2004) Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 74:969–985
Yang F, Lim GP, Begum AN et al (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901
Grundman M, Grundman M, Delaney P (2002) Antioxidant strategies for Alzheimer’s disease. Proc Nutr Soc 61:191–202
Ringman JM, Frautschy SA, Cole GM et al (2005) A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res 2:131–136
Singh D, Gupta M, Kesharwani R et al (2014) Molecular drug targets and therapies for Alzheimer’s disease. Transl Neurosci 5:203–217
Kim SJ, Son TG, Park HR et al (2008) Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem 283:14497–14505
Priyadarsini KI, Maity DK, Naik GH et al (2003) Role of phenolic O–H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radical Biol Med 35:475–484
Xu Y, Ku B, Cui L et al (2007) Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1 A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res 1162:9–18
Mythri RB, Bharath MM (2012) Curcumin: a potential neuroprotective agent in Parkinson’s disease. Curr Pharm Des 18:91–99
Mythri RB, Harish G, Dubey SK et al (2011) Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem 347:135–143
Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer 41:1955–1968
Garcea G, Jones DJ, Singh R et al (2004) Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 90:1011–1015
Pan MH, Huang, Lin JK (1999) Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 27:486–494
Anand P, Kunnumakkara AB, Newman RA et al (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818
Gupta NK, Dixit VK (2011) Development and evaluation of vesicular system for curcumin delivery. Arch Dermatol Res 303:89–101
Gupta NK, Dixit VK (2011) Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J Pharm Sci 100:1987–1995
Li C, Zhang Y, Su T et al (2012) Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int J Nanomedicine 7:5995–6002
Li H, Zhang N, Hao Y et al (2014) Formulation of curcumin delivery with functionalized single-walled carbon nanotubes: characteristics and anticancer effects in vitro. Drug Deliv 21:379–387
Ray B, Bisht S, Maitra A et al (2011) Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc) in the neuronal cell culture and animal model: implications for Alzheimer’s disease. J Alzheimers Dis 23:61–77
Bisht S, Feldmann G, Soni S et al (2007) Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 5:3
Tiyaboonchai W, Tungpradit W, Plianbangchang P (2007) Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm 337:299–306
Iwunze MO, McEwan D (2004) Peroxynitrite interaction with curcumin solubilized in ethanolic solution. Cell Mol Biol (Noisy-le-grand) 50: 749–752
Leung MH, Colangelo H, Kee TW (2008) Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir 24:5672–5675
Wang F, Wu X, Wang F et al (2006) The sensitive fluorimetric method for the determination of curcumin using the enhancement of mixed micelle. J Fluoresc 16:53–59
Ma Z, Haddadi A, Molavi O et al (2008) Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A 86:300–310
Sahu A, Bora U, Kasoju N et al (2008) Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater 4:1752–1761
Liu A, Lou H, Zhao L et al (2006) Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal 40:720–727
Bishnoi M, Chopra K, Rongzhu L et al (2011) Protective effect of curcumin and its combination with piperine (bioavailability enhancer) against haloperidol-associated neurotoxicity: cellular and neurochemical evidence. Neurotox Res 20:215–225
Suresh D, Srinivasan K (2010) Tissue distribution and elimination of capsaicin, piperine and curcumin following oral intake in rats. Indian J Med Res 131:682–691
Harish G, Venkateshappa C, Mythri RB et al (2010) Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: implications for Parkinson’s disease. Bioorg Med Chem 18:2631–2638
Furniss BS (1989) Vogel’s textbook of practical organic chemistry. (Pearson Education India), 5th edn. Longman Ltd., Harlow, p 654
Mohammed AI, Jwad RS (2011) J Kerbala Univ 9:68687
Joshi VY, Sawant MR (2006) A convenient stereoselective synthesis of beta-d-glucopyranosides. Indian J Chem 45:461–465
Mohri K, Watanabe Y, Yoshida Y et al (2003) Synthesis of glycosylcurcuminoids. Chem Pharm Bull (Tokyo) 51:1268–1272
Hergenhahn M, Bertram B, Wiessler M et al (2003) Curcumin derivatives with improved water solubility compared to curcumin and medicaments containing the same. US Patent Application No. 10/312,951
Vali S, Mythri RB, Jagatha B et al (2007) Integrating glutathione metabolism and mitochondrial dysfunction with implications for Parkinson’s disease: a dynamic model. Neuroscience 149:917–930
Wroblewski F, Ladue JS (1955) Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med 90:210–213
Asai A, Miyazawa T (2000) Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci 67:2785–2793
Ramadasan-Nair R, Gayathri N, Mishra S et al (2014) Mitochondrial alterations and oxidative stress in an acute transient mouse model of muscle degeneration: implications for muscular dystrophy and related muscle pathologies. J Biol Chem 289:485–509
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal BioChemistry 95:351–358
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Butterfield DA, Stadtman ER (1997) Protein oxidation processes in aging brain. Adv Cell Aging Gerontol 2:161–191
Mythri RB, Jagatha B, Pradhan N et al (2007) Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria? Antioxid Redox Signal 9:399–408
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Singh NP, McCoy MT, Tice RR et al (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Trounce IA, Kim YL, Jun AS et al (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509
Pandareesh MD, Anand T (2013) Neuromodulatory propensity of Bacopa monniera against scopolamine-induced cytotoxicity in PC12 cells via down-regulation of AChE and up-regulation of BDNF and muscarnic-1 receptor expression. Cell Mol Neurobiol 33:875–884
Pandareesh MD, Anand T (2014) Neuroprotective and anti-apoptotic propensity of Bacopa monniera extract against sodium nitroprusside induced activation of iNOS, heat shock proteins and apoptotic markers in PC12 cells. Neurochem Res 39:800–814
Hosamani R, Muralidhara (2009) Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology 30:977–985
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398
Linhart R, Wong SA, Cao J et al (2014) Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson’s disease mutant of Leucine-Rich Repeat Kinase 2 (LRRK2). Mol Neurodegener 9:23
Luo D, Zhang Q, Wang H et al (2009) Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur J Pharmacol 617:33–40
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bollimpelli VS, Kumar P, Kumari S et al (2016) Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem Int 95:37–45
Madathil KS, Karuppagounder SS, Haobam R et al (2013) Nitric oxide synthase inhibitors protect against rotenone-induced, oxidative stress mediated parkinsonism in rats. Neurochem Int 62:674–683
Dinkova-Kostova AT, Talalay P (2010) NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501:116–123
Ross D, Siegel D (2004) NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol 382:115–144
Martinez TN, Greenamyre JT (2012) Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid Redox Signal 16:920–934
Vassallo N (2008) Polyphenols and health: new and recent advances. Nova Publishers, New York
Prasad S, Tyagi AK, Aggarwal BB (2014) Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46:2–18
Sharma RA, Steward WP, Gescher AJ (2007) Pharmacokinetics and pharmacodynamics of curcumin. In: The molecular targets and therapeutic uses of curcumin in health and disease, pp 453–470
Gadad BS, Subramanya PK, Pullabhatla S et al (2012) Curcumin-glucoside, a novel synthetic derivative of curcumin, inhibits alpha-synuclein oligomer formation: relevance to Parkinson’s disease. Curr Pharm Des 18:76–84
Mishra S, Narain U, Mishra R et al (2005) Design, development and synthesis of mixed bioconjugates of piperic acid-glycine, curcumin-glycine/alanine and curcumin-glycine-piperic acid and their antibacterial and antifungal properties. Bioorg Med Chem 13:1477–1486
Liang C, Zhang Y, Jia Y et al (2016) Engineering a carbohydrate-processing transglycosidase into glycosyltransferase for natural product glycodiversification. Sci Rep 6:21051
Nataraj J, Manivasagam T, Justin Thenmozhi A et al (2016) Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr Neurosci 9:1–9
Sala G, Marinig D, Riva C et al (2016) Rotenone down-regulates HSPA8/hsc70 chaperone protein in vitro: A new possible toxic mechanism contributing to Parkinson’s disease. Neurotoxicology 54:161–169
Zhang Q, Chen S, Yu S et al (2016) Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson’s disease. Neuropharmacology 108:238–251
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Jagatha B, Mythri RB, Vali S et al (2008) Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radical Biol Med 44:907–917
Li N, Ragheb K, Lawler G et al (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525
Kelso GF, Porteous CM, Coulter CV et al (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276:4588–4596
Yu S, Zheng W, Xin N et al (2010) Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res 13:55–64
Chen YR, Tan TH (1998) Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17:173–178
Schaeffer HJ, Weber MJ (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 19:2435–2444
Dinkova-Kostova AT, Talalay P (2000) Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radical Biol Med 29:231–240
Radjendirane V, Joseph P, Lee YH et al (1998) Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem 273:7382–7389
Asher G, Tsvetkov P, Kahana C et al (2005) A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes dev 19:316–321
Tsvetkov P, Asher G, Reiss V et al (2005) Inhibition of NAD(P)H:quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc Natl Acad Sci USA 102:5535–5540
Gaikwad A, Long DJ 2nd, Stringer JL et al (2001) In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem 276:22559–22564
Tsvetkov P, Adamovich Y, Elliott E et al (2011) E3 ligase STUB1/CHIP regulates NAD(P)H:quinone oxidoreductase 1 (NQO1) accumulation in aged brain, a process impaired in certain Alzheimer disease patients. J Biol Chem 286:8839–8845
Lee SC, Zhao ML, Hirano A et al (1999) Inducible nitric oxide synthase immunoreactivity in the Alzheimer disease hippocampus: association with Hirano bodies, neurofibrillary tangles, and senile plaques. J Neuropathol Exp Neurol 58:1163–1169
Luth HJ, Munch G, Arendt T (2002) Aberrant expression of NOS isoforms in Alzheimer’s disease is structurally related to nitrotyrosine formation. Brain Res 953:135–143
Iadecola C, Alexander M (2001) Cerebral ischemia and inflammation. Curr Opin Neurol 14:89–94
Park EM, Cho S, Frys K et al (2004) Interaction between inducible nitric oxide synthase and poly(ADP-ribose) polymerase in focal ischemic brain injury. Stroke 35:2896–2901
Coulom H, Birman S (2004) Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci 24:10993–10998
Beal MF (2003) Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol 53(Suppl 3):S39–47 (discussion S38–47)
Celotto AM, Palladino MJ (2005) Drosophila: a “model” model system to study neurodegeneration. Mol Interv 5:292–303
Acknowledgments
This study was supported by a research grant from the Department of Biotechnology (DBT), India (Grant No. BT/PR4908/MED/30/745/2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
M. D. Pandareesh and M. K. Shrivash have contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandareesh, M.D., Shrivash, M.K., Naveen Kumar, H.N. et al. Curcumin Monoglucoside Shows Improved Bioavailability and Mitigates Rotenone Induced Neurotoxicity in Cell and Drosophila Models of Parkinson’s Disease. Neurochem Res 41, 3113–3128 (2016). https://doi.org/10.1007/s11064-016-2034-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2034-6