Abstract
This investigation evaluated the utility of a physiologically based pharmacokinetic (PBPK) model, which incorporates model parameters representing key determinants of monoclonal antibody (mAb) target-mediated disposition, to predict, a priori, mAb disposition in plasma and in tissues, including tumors that express target antigens. Monte Carlo simulation techniques were employed to predict the disposition of two mAbs, 8C2 (as a non-binding control mouse IgG1 mAb) and T84.66 (a high-affinity murine IgG1 anti-carcinoembryonic antigen mAb), in mice bearing no tumors, or bearing colorectal HT29 or LS174T xenografts. Model parameters were obtained or derived from the literature. 125I-T84.66 and 125I-8C2 were administered to groups of SCID mice, and plasma and tissue concentrations were determined via gamma counting. The PBPK model well-predicted the experimental data. Comparisons of the population predicted versus observed areas under the plasma concentration versus time curve (AUC) for T84.66 were 95.4 ± 67.8 versus 84.0 ± 3.0, 1,859 ± 682 versus 2,370 ± 154, and 5,930 ± 1,375 versus 5,960 ± 317 (nM × day) at 1, 10, and 25 mg/kg in LS174T xenograft-bearing SCID mice; and 215 ± 72 versus 233 ± 30, 3,070 ± 346 versus 3,120 ± 180, and 7,884 ± 714 versus 7,440 ± 626 in HT29 xenograft-bearing mice. Model predicted versus observed 8C2 plasma AUCs were 312.4 ± 30 versus 182 ± 7.6 and 7,619 ± 738 versus 7,840 ± 24.3 (nM × day) at 1 and 25 mg/kg. High correlations were observed between the predicted median plasma concentrations and observed median plasma concentrations (r 2 = 0.927, for all combinations of treatment, dose, and tumor model), highlighting the utility of the PBPK model for the a priori prediction of in vivo data.

















Similar content being viewed by others
References
Weiner LM, Surana R, Wang S (2010) Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 10:317–327
Yan L, Hsu K, Beckman RA (2008) Antibody-based therapy for solid tumors. Cancer J 14:178–183
Jain RK (1990) Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 50:814s–819s
Jain RK (1990) Tumor physiology and antibody delivery. Front Radiat Ther Oncol 24:32–46; discussion 64–38
Fujimori K, Covell DG, Fletcher JE, Weinstein JN (1990) A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med 31:1191–1198
Van Osdol W, Fujimori K, Weinstein JN (1991) An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res 51:4776–4784
Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN (1992) Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res 52:5144–5153
Sagan S, Charpentier S, Delfour A, Amiche M, Nicolas P (1992) The aspartic acid in deltorphin I and dermenkephalin promotes targeting to delta-opioid receptor independently of receptor binding. Biochem Biophys Res Commun 187:1203–1210
Jain RK, Weissbrod JM, Wei J (1980) Mass transport in tumors: characterization and applications to chemotherapy. Adv Cancer Res 33:251–310
Levy G (1994) Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 56:248–252
Schmidt MM, Thurber GM, Wittrup KD (2008) Kinetics of anti-carcinoembryonic antigen antibody internalization: effects of affinity, bivalency, and stability. Cancer Immunol Immunother 57:1879–1890
Weinstein JN, Eger RR, Covell DG, Black CD, Mulshine J, Carrasquillo JA, Larson SM, Keenan AM (1987) The pharmacology of monoclonal antibodies. Ann N Y Acad Sci 507:199–210
Fujimori K, Covell DG, Fletcher JE, Weinstein JN (1989) Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab’)2, and Fab in tumors. Cancer Res 49:5656–5663
Weinstein JN, van Osdol W (1992) Early intervention in cancer using monoclonal antibodies and other biological ligands: micropharmacology and the “binding site barrier”. Cancer Res 52:2747s–2751s
Fracasso G, Colombatti M (2000) Effect of therapeutic macromolecules in spheroids. Crit Rev Oncol Hematol 36:159–178
Ackerman ME, Pawlowski D, Wittrup KD (2008) Effect of antigen turnover rate and expression level on antibody penetration into tumor spheroids. Mol Cancer Ther 7:2233–2240
Thurber GM, Wittrup KD (2008) Quantitative spatiotemporal analysis of antibody fragment diffusion and endocytic consumption in tumor spheroids. Cancer Res 68:3334–3341
Graff CP, Wittrup KD (2003) Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res 63:1288–1296
Thurber GM, Zajic SC, Wittrup KD (2007) Theoretic criteria for antibody penetration into solid tumors and micrometastases. J Nucl Med 48:995–999
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54:1517–1528
Ferl GZ, Wu AM, DiStefano JJ 3rd (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33:1640–1652
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709
Urva SR, Yang VC, Balthasar JP (2010) Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci 99:1582–1600
Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2, and Fab’ in mice. Cancer Res 46:3969–3978
Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH (2008) A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol 8:401–413
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39:67–86
Chen J, Balthasar JP (2007) Development and characterization of high affinity anti-topotecan IgG and Fab fragments. In: Gad SC (ed) Handbook of pharmaceutical biotechnology. Wiley, Hoboken, pp 835–850
Abuqayyas L, Balthasar JP (2012) Pharmacokinetic mAb–mAb Interaction: Anti-VEGF mAb decreases the distribution of anti-CEA mAb into colorectal tumor xenografts. AAPS J 14(3):445–455
Nedelman JR, Gibiansky E, Lau DT (1995) Applying Bailer’s method for AUC confidence intervals to sparse sampling. Pharm Res 12:124–128
Nedelman JR, Jia X (1998) An extension of Satterthwaite’s approximation applied to pharmacokinetics. J Biopharm Stat 8:317–328
Butler TP, Grantham FH, Gullino PM (1975) Bulk transfer of fluid in the interstitial compartment of mammary tumors. Cancer Res 35:3084–3088
Greiner JW, Ullmann CD, Nieroda C, Qi CF, Eggensperger D, Shimada S, Steinberg SM, Schlom J (1993) Improved radioimmunotherapeutic efficacy of an anticarcinoma monoclonal antibody (131I-CC49) when given in combination with gamma-interferon. Cancer Res 53:600–608
Guadagni F, Witt PL, Robbins PF, Schlom J, Greiner JW (1990) Regulation of carcinoembryonic antigen expression in different human colorectal tumor cells by interferon-gamma. Cancer Res 50:6248–6255
Esteban JM, Kuhn JA, Felder B, Wong JY, Battifora H, Beatty JD, Wanek PM, Shively JE (1991) Carcinoembryonic antigen expression of resurgent human colon carcinoma after treatment with therapeutic doses of 90Y-alpha-carcinoembryonic antigen monoclonal antibody. Cancer Res 51:3802–3806
Shi ZR, Tsao D, Kim YS (1983) Subcellular distribution, synthesis, and release of carcinoembryonic antigen in cultured human colon adenocarcinoma cell lines. Cancer Res 43:4045–4049
Berk DA, Yuan F, Leunig M, Jain RK (1997) Direct in vivo measurement of targeted binding in a human tumor xenograft. Proc Natl Acad Sci USA 94:1785–1790
Wagner HE, Toth CA, Steele GD Jr, Thomas P (1992) Metastatic potential of human colon cancer cell lines: relationship to cellular differentiation and carcinoembryonic antigen production. Clin Exp Metastasis 10:25–31
Friedman J, Seger M, Levinsky H, Allalouf D (1987) Modulation of carcinoembryonic antigen release by HT-29 colon carcinoma line in the presence of different agents. Experientia 43:1121–1122
Hefta LJ, Neumaier M, Shively JE (1998) Kinetic and affinity constants of epitope specific anti-carcinoembryonic antigen (CEA) monoclonal antibodies for CEA and engineered CEA domain constructs. Immunotechnology 4:49–57
Wagener C, Clark BR, Rickard KJ, Shively JE (1983) Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: determination of affinities and specificities of monoclonal antibodies by using biotin-labeled antibodies and avidin as precipitating agent in a solution phase immunoassay. J Immunol 130:2302–2307
Rowlinson-Busza G, Maraveyas A, Epenetos AA (1995) Effect of tumour necrosis factor on the uptake of specific and control monoclonal antibodies in a human tumour xenograft model. Br J Cancer 71:660–665
Shah DK, Shin BS, Veith J, Toth K, Bernacki RJ, Balthasar JP (2009) Use of an anti-vascular endothelial growth factor antibody in a pharmacokinetic strategy to increase the efficacy of intraperitoneal chemotherapy. J Pharmacol Exp Ther 329:580–591
Pastuskovas CV, Mundo EE, Williams SP, Nayak TK, Ho J, Ulufatu S, Clark S, Ross S, Cheng E, Parsons-Reponte K, Cain G, Van Hoy M, Majidy N, Bheddah S, Dela Cruz Chuh J, Kozak KR, Lewin-Koh N, Nauka P, Bumbaca D, Sliwkowski M, Tibbitts J, Theil FP, Fielder PJ, Khawli LA, Boswell CA (2012) Effects of Anti-VEGF on Pharmacokinetics, Biodistribution, and Tumor Penetration of Trastuzumab in a Preclinical Breast Cancer Model. Mol Cancer Ther 11:752–762
Topp EM, Kitos PA, Vijaykumar V, DeSilva BS, Hendrickson TL (1998) Antibody transport in cultured tumor cell layers. J Control Release 53:15–23
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249–257
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7:987–989
US Food and Drug Administration (FDA) (2011) Label for Avastin (bevacizumab). http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0168lbl.pdf. Accessed April 2011
Jain RK (1987) Transport of molecules in the tumor interstitium: a review. Cancer Res 47:3039–3051
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41:189–207
Boswell CA, Ferl GZ, Mundo EE, Bumbaca D, Schweiger MG, Theil FP, Fielder PJ, Khawli LA (2011) Effects of anti-VEGF on predicted antibody biodistribution: roles of vascular volume, interstitial volume, and blood flow. PLoS One 6:e17874
Abraham AK, Krzyzanski W, Mager DE (2007) Partial derivative-based sensitivity analysis of models describing target-mediated drug disposition. AAPS J 9:E181–E189
Acknowledgments
This work was supported by funding from the Center for Protein Therapeutics and from the National Cancer Institute of the National Institutes of Health (CA114612).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
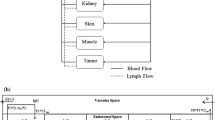
Model equations
-
1.
Plasma
$$ \begin{aligned} V_{p} \frac{{dC_{plasma} }}{dt} = (Q_{lung} - L_{lung} ) \times C_{lung}^{V} - (L_{gut} + L_{spleen} + Q_{liver} + Q_{heart} + Q_{kidney} + Q_{skin} + Q_{muscle} \\ & + Q_{tumor} ) \times C_{plasma} + \left( {\frac{1}{Tau} \times X_{lymph} } \right) \quad \quad \quad \,C_{plasma} (0) = \frac{Dose}{{V_{p} }} \\ \end{aligned} $$(1) -
2.
Lung
-
2.1.
Vascular space
$$ \begin{aligned} V_{lung}^{V} \frac{{dc_{lung}^{V} }}{dt} = (Q_{liver} - L_{liver} ) \times C_{liver}^{V} + (Q_{heart} - L_{heart} ) \times C_{heart}^{V} + (Q_{kidney} - L_{kidney} ) \times C_{kidney}^{V} \\ & + (Q_{skin} - L_{skin} ) \times C_{skin}^{V} + (Q_{muscle} - L_{muscle} ) \times C_{muscle}^{V} + (Q_{tumor} - L_{tumor} ) \\ & \times C_{tumor}^{V} + ( FR \times R1 \times (1 - fu_{lung} ) \times C_{lung}^{E,total} \times V_{lung}^{E} ) - (Q_{lung} - L_{lung} ) \times C_{lung}^{V} \\ & - ( R1 \times C_{lung}^{V} \times V_{lung}^{V} ) - ((1 - \sigma_{lung}^{V} ) \times L_{lung} \times C_{lung}^{V} ) \\ \end{aligned} $$(2) -
2.2.
Endothelial space
$$ \begin{aligned} V_{lung}^{E} \frac{{dc_{lung}^{E,total} }}{dt} = \left( { R1 \times C_{lung}^{V} \times V_{lung}^{V} } \right) - \left( {fu_{lung} \times CL_{lung} \times C_{lung}^{E,total} } \right) \\ & - \left( {\left( {1 - fu_{lung} } \right) \times R1 \times C_{lung}^{E,total} \times V_{lung}^{E} } \right) + \left( {R1 \times C_{lung}^{IS} \times V_{lung}^{IS} } \right) \\ \end{aligned} $$(3)
-
2.1.
-
2.3.
Interstitial space
$$ \begin{aligned} V_{lung}^{IS} \frac{{dc_{lung}^{IS,total} }}{dt} = \left( {\left( {1 - \sigma_{lung}^{V} } \right) \times L_{lung} \times C_{lung}^{V} } \right) + \left( {\left( {1 - FR} \right) \times R1 \times \left( {1 - fu_{lung} } \right) \times C_{lung}^{E,total} \times V_{lung}^{E} } \right) \\ & - \left( {\left( {1 - \sigma_{lung}^{L} } \right) \times L_{lung} \times C_{lung}^{IS} } \right) - R1 \times C_{lung}^{IS} \times V_{lung}^{IS} \\ \end{aligned} $$(4) -
3.
Liver
-
3.1.
Vascular space
$$ \begin{aligned} V_{liver}^{V} \frac{{dc_{liver}^{V} }}{dt} = (Q_{gut} - L_{gut} ) \times C_{gut}^{V} + (Q_{spleen} - L_{spleen} ) \times C_{spleen}^{V} + ((Q_{liver} - Q_{gut} - Q_{spleen} + L_{gut} \\ & + L_{spleen} ) \times C_{plasma} ) - (Q_{liver} - L_{liver} ) \times C_{liver}^{V} + (FR \times R1 \times (1 - fu_{liver} ) \times C_{liver}^{E,total} \\ & \times V_{liver}^{E} ) - ( R1 \times C_{liver}^{V} \times V_{liver}^{V} ) - ((1 - \sigma_{liver}^{V} ) \times L_{liver} \times C_{liver}^{V} ) \\ \end{aligned} $$(5)
-
3.2.
Endothelial space
$$ \begin{aligned} V_{lung}^{E} \frac{{dc_{lung}^{E,total} }}{dt} = \left( { R1 \times C_{liver}^{V} \times V_{liver}^{V} } \right) - \left( {fu_{liver} \times CL_{liver} \times C_{liver}^{E,total} } \right) \\ & - \left( {\left( {1 - fu_{liver} } \right) \times R1 \times C_{liver}^{E,total} \times V_{lung}^{E} } \right) + \left( {R1 \times C_{liver}^{IS} \times V_{liver}^{IS} } \right) \\ \end{aligned} $$(6)
-
3.3.
Interstitial space
$$ \begin{aligned} V_{liver}^{IS} \frac{{dc_{liver}^{IS} }}{dt} = \left( {\left( {1 - \sigma_{liver}^{V} } \right) \times L_{liver} \times C_{liver}^{V} } \right) + \left( {\left( {1 - FR} \right) \times R1 \times \left( {1 - fu_{liver} } \right) \times C_{liver}^{E,total} \times V_{liver}^{E} } \right) \\ & - \left( {\left( {1 - \sigma_{liver}^{L} } \right) \times L_{liver} \times C_{liver}^{IS} } \right) - R1 \times C_{liver}^{IS} \times V_{liver}^{IS} \\ \end{aligned} $$(7)
-
3.1.
-
4.
Tumor
-
4.1.
Vascular space
$$ \begin{aligned} V_{tumor}^{V} \frac{{dc_{tumor}^{V} }}{dt} = Q_{tumor} \times C_{plasma} - (Q_{tumor} - L_{tumor} ) \times C_{tumor}^{V} + (FR \times R1 \times (1 - fu_{tumor} ) \times C_{tumor}^{E,total} \\ & \times V_{tumor}^{E} ) - ( R1 \times C_{tumor}^{V} \times V_{tumor}^{V} ) - ((1 - \sigma_{tumor}^{V} ) \times L_{tumor} \times C_{tumor}^{V} ) \\ \end{aligned} $$(8) -
4.2.
Endothelial space
$$ \begin{aligned} V_{tumor}^{E} \frac{{dc_{tumor}^{E,total} }}{dt} = \left( { R1 \times C_{tumor}^{V} \times V_{tumor}^{V} } \right) - \left( {fu_{tumor} \times CL_{tumor} \times C_{tumor}^{E,total} } \right) \\ & - \left( {\left( {1 - fu_{tumor} } \right) \times R1 \times C_{tumor}^{E,total} \times V_{tumor}^{E} } \right) + \left( {R1 \times fu_{tumor}^{IS,cell} \times C_{tumor}^{IS} \times V_{tumor}^{IS} } \right) \\ \end{aligned} $$(9) -
4.3.
Interstitial space
$$ \begin{aligned} V_{{tumor}}^{{IS}} \frac{{dc_{{tumor}}^{{IS}} }}{{dt}} & = \left( {(1 - \sigma _{{tumor}}^{V} ) \times L_{{tumor}} \times C_{{tumor}}^{V} } \right) + \left( {(1 - FR) \times R1 \times (1 - fu_{{tumor}} ) \times C_{{tumor}}^{{E,total}} \times V_{{tumor}}^{E} } \right) \\ & - \left( {(1 - \sigma _{{tumor}}^{L} ) \times L_{{tumor}} \times fu_{{tumor}}^{{IS,cell}} \times C_{{tumor}}^{{IS}} } \right) - R1 \times fu_{{tumor}}^{{IS,cell}} \times C_{{tumor}}^{{IS}} \times V_{{tumor}}^{{IS}} - (1 - fu_{{tumor}}^{{IS,cell}} ) \times C_{{tumor}}^{{IS}} \\ & \times k_{int} \times V_{{tumor}}^{{IS}} \\ \end{aligned} $$(10)
Please note: The model assumes an instantaneous binding equilibrium between unbound mAb and mAb in complex with tumor antigens. Binding to the target receptor influences the rate of mass loss from the interstitial space via convection (through influence on \( fu_{tumor}^{IS,cell} \)) and via target-mediated clearance (via influence on \( (1 - fu_{tumor}^{IS,cell} ) \)).
-
4.1.
-
5.
Other organs (i)
-
5.1.
Vascular space
$$ \begin{aligned} V_{i}^{V} \frac{{dc_{i}^{V} }}{dt} = Q_{i} \times C_{plasma} - (Q_{i} - L_{i} ) \times C_{i}^{V} + ( FR \times R1 \times (1 - fu_{i} ) \times C_{i}^{E,total} \times V_{i}^{E} ) - ( R1 \times C_{i}^{V} \times V_{i}^{V} ) \\ & - ((1 - \sigma_{i}^{V} ) \times L_{i} \times C_{i}^{V} ) \\ \end{aligned} $$(11) -
5.2
Endothelial space
$$ \begin{aligned} V_{i}^{E} \frac{{dc_{i}^{E,total} }}{dt} = \left( { R1 \times C_{i}^{V} \times V_{i}^{V} } \right) - \left( {fu_{i} \times CL_{i} \times C_{i}^{E,total} } \right) - \left( {\left( {1 - fu_{i} } \right) \times R1 \times C_{i}^{E,total} \times V_{i}^{E} } \right) \\ & + \left( {R1 \times C_{i}^{IS} \times V_{i}^{IS} } \right) \\ \end{aligned} $$(12) -
5.3.
Interstitial space
$$ \begin{aligned} V_{i}^{IS} \frac{{dc_{i}^{IS} }}{dt} = \left( {\left( {1 - \sigma_{i}^{V} } \right) \times L_{i} \times C_{i}^{V} } \right) + \left( {\left( {1 - FR} \right) \times R1 \times \left( {1 - fu_{i} } \right) \times C_{i}^{E,total} \times V_{i}^{E} } \right) \\ & - \left( {\left( {1 - \sigma_{i}^{L} } \right) \times L_{i} \times C_{i}^{IS} } \right) - R1 \times C_{i}^{IS} \times V_{i}^{IS} \\ \end{aligned} $$(13)
-
5.1.
-
6.
Lymph node
$$ \frac{{dX_{lymph} }}{dt} = \left( {\mathop \sum \nolimits (1 - \sigma_{i}^{l} ) \times L_{i} \times C_{i}^{IS} )} \right) + \left( {1 - \sigma_{tumor}^{l} } \right) \times L_{tumor} \times fu_{tumor}^{IS,cell} \times C_{tumor}^{IS} - \frac{1}{Tau} \times X_{lymph} $$(14) -
7.
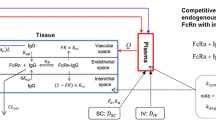
For each organ, the \( fu_{i} \) to organ FcRn in the endosomal space was described as follow:
$$ fu_{i} = 1 - \frac{1}{{2 \times C_{i}^{E,total} }} \times \left[ {\left( {K_{d}^{FcRn} + nPt_{i} + C_{i}^{E,total} } \right) - \sqrt {\left( {K_{d}^{FcRn} + nPt_{i} + C_{i}^{E,total} } \right)^{2} - \left( {4 \times nPt_{i} \times C_{i}^{E,total} } \right)} } \right] $$(15)$$ C_{i}^{E,total} = C_{i}^{E,endogenous} + C_{i}^{E,exogenous} $$(16)For tumor compartment, the \( fu_{tumor}^{IS,cell} \) to tumor antigens in the interstitial space was described as follow:
$$ \begin{aligned} fu_{{tumor}}^{{IS,cell}} = & 1 - \frac{1}{{2 \times C_{{tumor}}^{{IS,total}} }} \\ \times & \left[ {\left( {K_{{d_{{tumor}} }}^{{CEA}} + FA \times nPt_{{tumor}}^{{CEA}} + C_{{tumor}}^{{IS}} } \right)} \right] \\ - & \sqrt {\left( {K_{{d_{{tumor}} }}^{{CEA}} + FA \times nPt_{{tumor}}^{{CEA}} + C_{{tumor}}^{{IS,total}} } \right)^{2} - \left( {4 \times FA \times nPt_{{tumor}}^{{CEA}} + C_{{tumor}}^{{IS,total}} } \right)} \\ \end{aligned} $$(17) -
8.
Total antibody concentration was calculated as follow
$$ C_{i}^{total} = \frac{{C_{i}^{V} \times V_{i}^{V} + C_{i}^{E} \times V_{i}^{E} + C_{i}^{IS} \times V_{i}^{IS} }}{{V_{i}^{total} }} $$(18)For tumor compartment total tumor volume was defined using an exponential growth function, which represents the sum of all sub-compartments
$$ V_{tumor}^{total} = V_{tumor}^{V} + V_{tumor}^{E} + V_{tumor}^{IS} + V_{tumor}^{Cellular} $$(19)
Rights and permissions
About this article
Cite this article
Abuqayyas, L., Balthasar, J.P. Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn 39, 683–710 (2012). https://doi.org/10.1007/s10928-012-9279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9279-8