Abstract
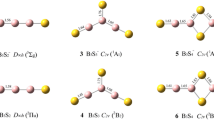
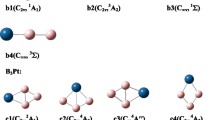
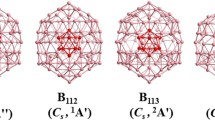
A systematic density functional theory and wave function theory investigation performed in this work reveals a planar-to-icosahedral structural transition between n = 4–5 in the partially hydrogenated B12H 0/− n clusters (n = 1–6) upon hydrogenation of all-boron B 0/−12 . Coupled cluster calculations with triple excitations (CCSD(T)) indicate that a distorted icosahedral B12H6 cluster with C2 symmetry is overwhelmingly favored (by 35 kcal/mol) over the recently proposed perfectly planar borozene (D3h B12H6) (Szwacki et al., Nanoscale Res Lett 4:1085, 2009) which proves to be a high-lying local minimum. A similar 2D–3D structural transition occurs to the corresponding boron boronyl analogues of B12(BO) n with n –BO terminals. Detailed adaptive natural density partitioning (AdNDP) analyses reveal the bonding patterns of these quasi-planar or cage-like clusters which are characterized with delocalized σ and π molecular orbitals. The electron detachment energies of the concerned anions and excitation energies of the neutrals are also predicted to facilitate their future experimental characterizations.





Similar content being viewed by others
References
F. A. Cotton, G. Wilkinson, C. A. Murrillo, and M. Bochmann Advanced Inorganic Chemistry, 6th ed (John Wiley & Sons, New York, 1999).
A. N. Alexandrova, A. I. Boldyrev, H. J. Zhai, and L. S. Wang (2006). Coord. Chem. Rev. 250, 2811.
M. Y. Zubarev and A. I. Boldyrev (2007). J. Comput. Chem. 28, 251.
H.-J. Zhai, B. Kiran, J. Li, and L. S. Wang (2003). Nat. Mater. 2, 827.
B. Kiran, S. Bulusu, H. J. Zhai, S. Yoo, X. C. Zeng, and L. S. Wang (2005). Proc. Natl Acad. Sci. USA 102, 961.
A. P. Sergeeva, D. Y. Zubarev, H. J. Zhai, A. I. Boldyrev, and L. S. Wang (2008). J. Am. Chem. Soc. 130, 7244.
W. Huang, A. P. Sergeeva, H. J. Zhai, B. B. Averkiev, L. S. Wang, and A. I. Boldyrev (2010). Nat. Chem. 2, 202.
A. P. Sergeeva, B. B. Averkiev, H. J. Zhai, A. I. Boldyrev, and L. S. Wang (2011). J. Chem. Phys. 134, 224304.
N. G. Szwacki, V. Weber, and C. J. Tymczak (2009). Nanoscale Res. Lett. 4, 1085.
G. Forte, A. La Magna, I. Deretzis, and R. Pucci (2010). Nanoscale Res. Lett. 5, 158.
S. Sahu and A. Shukla (2010). Nanoscale Res. Lett. 5, 714.
N. G. Szwacki (2008). Nanoscale Res. Lett. 3, 49.
A. N. Alexandrova, E. Koyle, and A. I. Boldyrev (2006). J. Mol. Model. 12, 569.
M. Boyukata, C. Ozdogan, and Z. B. Guvenc (2007). J. Mol. Struct. (THEOCHEM) 805, 91.
A. N. Alexandrova, K. A. Birch, and A. I. Boldyrev (2003). J. Am. Chem. Soc. 125, 10786.
Y. Ohishi, K. Kimura, M. Yamaguchi, N. Uchida, and T. Kanayama (2008). J. Chem. Phys. 128, 124304.
D. Y. Zubarev and A. I. Boldyrev (2008). Phys. Chem. Chem. Phys. 10, 5207.
D. Y. Zubarev and A. I. Boldyrev (2008). J. Org. Chem. 73, 9251.
D. Y. Zubarev and A. I. Boldyrev (2009). J. Phys. Chem. A 113, 866.
Q. Chen and S. D. Li (2011). J. Clust. Sci. doi:10.1007/s10876–011-0400–8.
Q. Chen, H. Bai, J. C. Guo, C. Q. Miao, and S. D. Li (2011). Phys. Chem. Chem. Phys., submitted.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785.
M. Head-Gordon, J. A. Pople, and M. Frisch (1988). Chem. Phys. Lett. 153, 503.
M. Head-Gordon and T. Head-Gordon (1994). Chem. Phys. Lett. 220, 122.
J. A. Pople, M. Head-Gordon, and K. Raghavachari (1987). J. Chem. Phys. 87, 5968.
G. E. Scuseria and H. F. Schaefer III (1989). J. Chem. Phys. 90, 3700.
G. E. Scuseria, C. L. Janssen, and H. F. Schaefer III (1988). J. Chem. Phys. 89, 7382.
J. Cizek (1969). Adv. Chem. Phys. 14, 35.
G. Schaftenaar, MOLDEN, version 4.1 (Centre for Molecular and Biomolecular Informatics (CMBI), Nijmegen, 2003).
P. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, and N. J. R. van Eikema Hommes (1996). J. Am. Chem. Soc. 118, 6317.
H. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta, and P. R. Schleyer (2006). Org. Lett. 8, 863.
K. Wolinski, J. F. Hilton, and P. Pulay (1990). J. Am. Chem. Soc. 112, 8251.
M. J. Frisch, et al. Gaussian 03, Revision, A. 1 (Gaussian, Inc., Pittsburgh, PA, 2003).
H. J. Zhai, M. Wang, S. D. Li, and L. S. Wang (2007). J. Phys. Chem. A 111, 1030.
H. J. Zhai, S. D. Li, and L. S. Wang (2007). J. Am. Chem. Soc. 129, 9254.
S. D. Li, H. J. Zhai, and L. S. Wang (2008). J. Am. Chem. Soc. 130, 2573.
H. Tang and S. Ismail-Beigi (2007). Phys. Rev. Lett. 99, 115501.
H. Tang and S. Ismail-Beigi (2009). Phys. Rev. B. 80, 134113.
Acknowledgments
This work was jointly supported by the National Science Foundation of China (No. 20873117) and Shanxi Natural Science Foundation (No. 2010011012-3). The authors are grateful to Professor A. I. Boldyrev and Dr. T. Galeev and A. Sergeeva at Utah State University for their generous help in using the AdNDP program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, H., Li, SD. Hydrogenation of B 0/−12 : A Planar-to-Icosahedral Structural Transition in B12H 0/− n (n = 1–6) Boron Hydride Clusters. J Clust Sci 22, 525–535 (2011). https://doi.org/10.1007/s10876-011-0408-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-011-0408-0