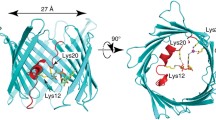
Abstract
The voltage-dependent anion channel (VDAC) is the most abundant protein of the outer mitochondrial membrane and constitutes the major pathway for the transport of ADP, ATP, and other metabolites. In this multidisciplinary study we combined solid-state NMR, electrophysiology, and molecular dynamics simulations, to study the structure of the human VDAC isoform 2 in a lipid bilayer environment. We find that the structure of hVDAC2 is similar to the structure of hVDAC1, in line with recent investigations on zfVDAC2. However, hVDAC2 appears to exhibit an increased conformational heterogeneity compared to hVDAC1 which is reflected in broader solid-state NMR spectra and less defined electrophysiological profiles.








Similar content being viewed by others
References
Anflous K, Armstrong DD, Craigen WJ (2001) Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J Biol Chem 276:1954–1960. doi:10.1074/jbc.M006587200
Bauer AJ, Gieschler S, Lemberg KM et al (2011) Functional model of metabolite gating by human voltage-dependent anion channel 2. Biochemistry 50:3408–3410. doi:10.1021/bi2003247
Bayrhuber M, Meins T, Habeck M et al (2008) Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA 105:15370–15375. doi:10.1073/pnas.0808115105
Benz R (1994) Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta 1197:167–196
Benz R, Janko K, Boos W, Läuger P (1978) Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta (BBA) 511:305–319
Benz R, Schmid A, Hancock R (1985) Ion selectivity of gram-negative bacterial porins. J Bacteriol 162:722–727
Berendsen HJC, Postma JPM, van Gunsteren WF et al (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684. doi:10.1063/1.448118
Berger O, Edholm O, Jähnig F (1997) Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys J 72:2002–2013. doi:10.1016/S0006-3495(97)78845-3
Blachly-Dyson E, Song J (1997) Multicopy suppressors of phenotypes resulting from the absence of yeast VDAC encode a VDAC-like protein. Mol Cell Biochem 17:5727–5738
Blachly-Dyson E, Zambronicz EB, Yu WH et al (1993) Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J Biol Chem 268:1835–1841
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:014101. doi:10.1063/1.2408420
Cheng EHY, Sheiko TV, Fisher JK et al (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301:513–517. doi:10.1126/science.1083995
Choudhary OP, Paz A, Adelman JL et al (2014) Structure-guided simulations illuminate the mechanism of ATP transport through VDAC1. Nat Struct Mol Biol 21:626–632. doi:10.1038/nsmb.2841
Colombini M (1980) Pore Size and Properties of Channels from Mitoehondria Isolated from Neurospora crassa. J MembrBiol 53:79–84
Colombini M (2004) VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem 256–257:107–115
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys 98:10089. doi:10.1063/1.464397
Daura X, Gademann K, Jaun B et al (1999) Peptide folding: when simulation meets experiment. Angew Chem Int Ed Engl 38:236–240. doi:10.1002/(SICI)1521-3773(19990115)38:1/2<236:AID-ANIE236>3.0.CO;2-M
De Pinto V, Tomasello F, Messina A et al (2007) Determination of the conformation of the human VDAC1 N-terminal peptide, a protein moiety essential for the functional properties of the pore. ChemBioChem 8:744–756. doi:10.1002/cbic.200700009
Engelhardt H, Meins T, Poynor M et al (2007) High-level expression, refolding and probing the natural fold of the human voltage-dependent anion channel isoforms I and II. J Membr Biol 216:93–105. doi:10.1007/s00232-007-9038-8
Feenstra KA, Hess B, Berendsen HJC (1999) Improving efficiency of large time-scale molecular dynamics simulations of hydrogen-rich systems. J Comput Chem 20:786–798. doi:10.1002/(SICI)1096-987X(199906)20:8<786:AID-JCC5>3.0.CO;2-B
Fung BM, Khitrina K, Ermolaev K (2000) An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson 142:97–101. doi:10.1006/jmre.1999.1896
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472. doi:10.1002/(SICI)1096-987X(199709)18:12<1463:AID-JCC4>3.0.CO;2-H
Hess B, Uppsala S, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation, pp 435–447
Hiller S, Garces RG, Malia TJ et al (2008) Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206–1210. doi:10.1126/science.1161302
Jorgensen W (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Jorgensen WL, Madura JD (1983) Solvation and conformation of methanol in water. J Am Chem Soc 105:1407–1413
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637. doi:10.1002/bip.360221211
Krammer E-M, Homblé F, Prévost M (2011) Concentration dependent ion selectivity in VDAC: a molecular dynamics simulation study. PLoS ONE 6:e27994. doi:10.1371/journal.pone.0027994
Lindén M, Gellerfors P (1983) Hydrodynamic properties of porin isolated from outer membranes of rat liver mitochondria. Biochim Biophys Acta 736:125–129
Miyamoto S, Kollman P (1992) Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem 13:952–962. doi:10.1002/jcc.540130805
Naghdi S, Varnai P, Hunyady L, Hajnoczky G (2012) The isoform specific N terminus of VDAC2 is dispensable for tBid induced cytochrome C release. Biophys J 102:437a. doi:10.1016/j.bpj.2011.11.2391
Popp B, Court DA, Benz R, Neupert W, Lill R (1996) The role of the N and C termini of recombinant Neurospora mitochondrial porin in channel formation and voltage-dependent gating. J Biol Chem 271:13593–13899
Reina S, Palermo V, Guarnera A et al (2010) Swapping of the N-terminus of VDAC1 with VDAC3 restores full activity of the channel and confers anti-aging features to the cell. FEBS Lett 584:2837–2844. doi:10.1016/j.febslet.2010.04.066
Rostovtseva T, Colombini M (1997) VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J 72:1954–1962. doi:10.1016/S0006-3495(97)78841-6
Rui H, Lee KI, Pastor RW, Im W (2011) Molecular dynamics studies of ion permeation in VDAC. Biophys J 100:602–610. doi:10.1016/j.bpj.2010.12.3711
Runke G, Maier E, Summers W et al (2006) Deletion variants of Neurospora mitochondrial porin: electrophysiological and spectroscopic analysis. Biophys J 90:3155–3164. doi:10.1529/biophysj.105.072520
Šali A, Blundell T (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Sampson MJ, Lovell RS, Craigen WJ (1996) Isolation, characterization, and mapping of two mouse mitochondrial voltage-dependent anion channel isoforms. Genomics 33:283–288. doi:10.1006/geno.1996.0193
Sampson MJ, Lovell RS, Craigen WJ (1997) The Murine voltage-dependent anion channel gene family. J Biol Chem 272:18966–18973
Sampson MJ, Decker WK, Beaudet AL et al (2001) Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem 276:39206–39212. doi:10.1074/jbc.M104724200
Schein SJ, Colombini M, Finkelstein A (1976) Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol 30:99–120
Schneider R, Etzkorn M, Giller K et al (2010) The native conformation of the human VDAC1 N terminus. Angew Chem Int Ed Engl 49:1882–1885. doi:10.1002/anie.200906241
Schredelseker J, Paz A, López CJ et al (2014) High resolution structure and double electron–electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J Biol Chem 289:12566–12577. doi:10.1074/jbc.M113.497438
Smack DP, Colombini M (2014) Voltage-dependent channels found in the membrane fraction of corn mitochondria. 1985:1094–1097
Sorgato MC, Moran O (1993) Channels in mitochondrial membranes: knowns, unknowns, and prospects for the future. Crit Rev Biochem Mol Biol 28:127–171
Teijido O, Rappaport SM, Chamberlin A et al (2014) Acidification affects voltage-dependent anion channel functioning asymmetrically: role of salt bridges. J Biol Chem. doi:10.1074/jbc.M114.576314
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Ujwal R, Cascio D, Colletier J-P et al (2008) The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA 105:17742–17747. doi:10.1073/pnas.0809634105
Villinger S, Briones R, Giller K et al (2010) Functional dynamics in the voltage-dependent anion channel. Proc Natl Acad Sci USA 107:22546–22551. doi:10.1073/pnas.1012310108
Villinger S, Giller K, Bayrhuber M (2014) Nucleotide interactions of the human voltage-dependent anion channel. J Biol Chem 289:13397–13406. doi:10.1074/jbc.M113.524173
Wang J, Wolf RM, Caldwell JW et al (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174. doi:10.1002/jcc.20035
Xu X, Decker W, Sampson M (1999) Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J Membr Biol 170:89–102
Yagoda N, von Rechenberg M, Zaganjor E et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:864–868. doi:10.1038/nature05859
Yamagata H, Shimizu S, Nishida Y et al (2009) Requirement of voltage-dependent anion channel 2 for pro-apoptotic activity of Bax. Oncogene 28:3563–3572. doi:10.1038/onc.2009.213
Yu T-Y, Raschle T, Hiller S, Wagner G (2012) Solution NMR spectroscopic characterization of human VDAC-2 in detergent micelles and lipid bilayer nanodiscs. Biochim Biophys Acta 1818:1562–1569. doi:10.1016/j.bbamem.2011.11.012
Zachariae U, Schneider R, Briones R et al (2012) β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure 20:1540–1549. doi:10.1016/j.str.2012.06.015
Acknowledgments
We thank B. Angerstein for expert technical assistance and R. Briones for help in the initial stages of the project concerning GROMACS. This work was supported by the Max Planck Society, the Leibniz-Institut für Molekulare Pharmakologie, the ERC (grant agreement number 282008 to M.Z.), the DFG (Collaborative research center 803 to A.L., C.G., and M.Z. and Emmy Noether Fellowship to A.L.) and by a Marie Curie fellowship within the 7th EU Framework Program to Z.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gattin, Z., Schneider, R., Laukat, Y. et al. Solid-state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J Biomol NMR 61, 311–320 (2015). https://doi.org/10.1007/s10858-014-9876-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-014-9876-5