Abstract
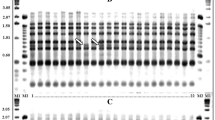
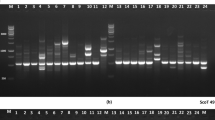
Wild annual Cicer gene pools contain valuable germplasm for chickpea improvement programs. Previous research showed that duplication might exist in accessions collected from these gene pools, which would hinder chickpea breeding and related research. AFLP (amplified fragment length polymorphism) markers were used to fingerprint the world collections of the primary and secondary gene pools including C. reticulatum Lad., C. bijugum K.H. Rech., C. judaicum Boiss. and C. pinnatifidum Jaub. et Sp. Duplicates were detected in a total of 24 accessions in both the gene pools, highlighting the necessity to fingerprint the germplasm. Genotypic difference was detected as gene pool specific, species specific and accession specific AFLP markers. These were developed into fingerprinting keys for accession identification between and within species and gene pools. Use of AFLP markers to detect duplicates and to identify accessions is a reliable method which will assist in the characterisation and use of wild annual Cicer germplasm in chickpea improvement programs. We recommend the procedure presented in this paper as a standard approach for the precise genetic identification and characterisation of future world collections of wild Cicer, to keep germplasm integrity and to benefit chickpea breeding and related research programs.
Similar content being viewed by others
References
Abbo S., Berger J. and Turner N.C. (2003). Evolution of cultivated chickpea: four bottlenecks limit diversity and constrain adaptation. Funct. Plant Biol. 30: 1081–1087
Arens P., Coops H., Jansen J. and Vosman B. (1998). Molecular genetic analysis of black poplar (Populus nigra L.) along Dutch rivers. Mol. Ecol. 7: 11–18
Astarini I.A., Plummer J.A., Lancaster R.A. and Yan G. (2004). Fingerprinting of cauliflower cultivars using RAPD markers. Aust. J. Agric. Res. 55: 117–124
Bekele E., Fido R.J., Tatham A.S. and Shewry P.R. (1995). Heterogeneity and polymorphism of seed proteins in tef (Eragrostis tef). Hereditas 122: 67–72
Berger J., Abbo S. and Turner N.C. (2003). Ecogeography of annual Cicer species: the poor state of the world collection. Crop Sci. 43: 1076–1090
Cabrita L.F., Aksoy U., Hepaksoy S. and Leitao J.M. (2001). Suitability of isozymeRAPD and AFLP markers to assess genetic differences and relatedness among fig (Ficus carica L.) clones. Sci. Hortic. 87: 261–273
Cansian R.L. and Echeverrigaray S. (2000). Discrimination among cultivars of cabbage using randomly amplified polymorphic DNA markers. Hortscience 35: 1155–1158
Choumane W., Winter P., Weigand F. and Kahl G. (2000). Theor. Appl. Genet. 101: 269–278
Clarke H.J., Kuo I., Kuo J. and Siddique K.H.M. (2004). Abortion and stages for embryo rescue following wide crosses between chickpea (Cicer arietinum L.) and C. bijugum K.H. Rech. In: (eds) Legumes for the Benefit of AgricultureNutrition and the environment (5th European Conference on Grain Legumes and 2nd International Conference on Legume Genomics and Genetics), 7–11 June 2004, pp 193. Dijon, France
Cooke R.J. (1999). Modern methods for cultivar verification and the transgenic plant challenge. Seed Sci. Technol. 27: 669–680
Croser J.S., Ahmad F., Clarke H.J. and Siddique K.H.M. (2003). Utilisation of wild Cicer in chickpea improvement - progress, constraints, and prospects. Aust. J. Agr. Res. 54: 429–444
Gillies A.C.M. and Abbott R.J. (1998). Evaluation of random amplified polymorphic DNA for species identification and phylogenetic analysis in Stylosanthes (Fabaceae). Plant Syst. Evol. 211: 201–216
Knights T., Brinsmead B., Fordyce M., Wood J., Kelly A. and Harden S. (2002). Use of the wild relative Cicer echinospermum in chickpea improvement. In: McComb, J.A. (eds) Proceedings of the 12th Australasian Plant Breeding Conference15–20th September, 2002, pp 150–154. Australasian Plant Breeding Assoc. Inc, Perth, W. Australia
Kumar P.P., Yau J.C.K. and Goh C.J. (1998). Genetic analyses of Heliconia species and cultivars with randomly amplified polymorphic DNA (RAPD) markers. J. Am. Soc. Hortic. Sci. 123: 91–97
Mallikarjuna N. (1999). Ovule and embryo culture to obtain hybrids from interspecific incompatible pollinations in chickpea. Euphytica 110: 1–6
Noli E., Conti S., Maccaferri M. and Sanguineti M.C. (1999). Molecular characterization of tomato cultivars. Seed Sci. Technol. 27: 1–10
Pradhan A., Yan G. and Plummer J.A. (2004). Development of DNA fingerprinting keys for the identification of radish cultivars. Aust. J. Exp. Agr. 44: 95–102
Robertson L.D., Singh K.B. and Ocampo B. (1995). A Catalog of Annual Wild Cicer Species. International Center for Agricultural Research in the Dry Areas (ICARDA). Aleppo, Syria
Shan F., Yan G. and Plummer J.A. (2003a). Karyotype evolution in the genus Boronia (Rutaceae). Bot. J. Linn. Soc. 142: 309–320
Shan F., Yan G. and Plummer J.A. (2003b). Meiotic chromosome behaviour and Boronia (Rutaceae) genome reorganization. Aust. J. Bot. 51: 599–607
Shan F., Yan G. and Plummer J.A. (2003c). Cytoevolution of Boronia genome revealed by flourescent in situ hybridisation with rDNA probes. Genome 46: 507–513
Shan F., Clarke H.J., Yan G., Plummer J.A. and Siddique K.H.M. (2004). Development of DNA fingerprinting keys for discrimination of Cicer echinospermum P.H. Davis accessions using AFLP markers. Aust. J. Agric. Res. 55: 947–952
Shan F., Clarke H.J., Plummer J.A., Yan G. and Siddique K.H.M. (2005). Geographical patterns of genetic variation in the world collections of wild annual Cicer characterized by amplified fragment length polymorphisms. Theor. Appl. Genet. 110: 381–391
Singh K.B., Ocampo B. and Robertson L.D. (1998). Diversity for abiotic and biotic stress resistance in the wild annual Cicer species. Genet. Resour. Crop Evol. 45: 9–17
Singh S., Gumber R.K., Joshi N. and Singh K. (2005). Introgression from wild Cicer reticulatum to cultivated chickpea for productivity and disease resistance. Plant Breeding 124: 477–480
Tohme J., Orlando Gonzalez D., Beebe S. and Duque M.C. (1996). AFLP analysis of gene pools of a wild bean core collection. Crop Sci. 36: 1375–1384
Mes T.H.M., den Jijs J.C.M. and Bachmann K. (2000). Amplified fragment length polymorphism (AFLP) markers reveal that population structure of triploid dandelions (Taraxacum officinale) exhibits both clonality and recombination. Mol. Ecol. 9: 1–8
Virk P.S., Newbury H.J., Jackson M.T. and Ford-Lloyd B.V. (1995). The identification of duplicate accessions within a rice germplasm collection using RAPD analysis. Theor. Appl. Genet. 90: 1049–1055
Winfield M.O., Arnold G.M., Cooper F., Le Ray M., White J., Karp A. and Edwards K.J. (1998). A study of genetic diversity in Populus nigra subsp. betulifolia in the upper Severn area of the UK using AFLP markers. Mol. Ecol. 7: 3–10
Winter P., Pfaff T., Udupa S.M., Huettel B., Sharma P.C., Sahi S., Arreguin-Espinoza R., Weigand F., Muehlbauer F.J. and Kahl G. (1999). Characterization and mapping of sequence-tagged microsatellite sites in the chickpea (Cicer arietinum L.) genome. Mol. Gen. Genet. 262: 90–101
Yadav S.S., Turner N.C. and Kumar J. (2002). Commercialization and utilization of wild genes for higher productivity in chickpea. In: McComb, J.A. (eds) Proceedings of the 12th Australasian Plant Breeding Conference15–20th September, 2002, pp 155–160. Australasian Plant Breeding Assoc. Inc, Perth, W. Australia
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shan, F., Clarke, H.J., Yan, G. et al. Identification of duplicates and fingerprinting of primary and secondary wild annual Cicer gene pools using AFLP markers. Genet Resour Crop Evol 54, 519–527 (2007). https://doi.org/10.1007/s10722-006-0008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-006-0008-2