Abstract
Background
Ubiquitin carboxyl-terminal hydrolase 37 (UCH37), a member of the DUBs, was found to play an important role in oncogenesis through promoting some Proto-oncogenes’ expression and stem cell-like characteristics in the cell in previous research. The aim of this study was to assess the value of UCH37 in predicting tumor recurrence after curative resection in esophageal squamous cell carcinoma (ESCC) patients.
Methods
We analyzed UCH37 protein expression in 111 clinicopathologically characterized ESCC cases, from those who underwent curative resection between 2007 and 2008, by immunohistochemistry. The prognostic significance was assessed using Kaplan–Meier survival estimates and log-rank tests.
Results
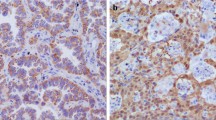
We found that UCH37 expression was higher in the cancer tissue than in non-tumorous control tissue at protein level and was overexpressed in tumor tissues of recurrent patients. There was a significant difference of UCH37 expression in patients categorized according to TNM stage (p = 0.038) and lymph nodes metastasis condition (p = 0.009). Univariate analyses revealed that UCH37 was a significant predictor for overall survival and disease-free survival, and multivariate analyses showed that UCH37 was an independent prognostic marker for ESCC recurrence. A prognostic significance of UCH37 was also found in the subgroup of lymph nodes metastasis condition classification. About 90 % of the recurrent patients recurred within 2 years, of which 84.4 % were predicted by UCH37.
Conclusion
UCH37 is associated with outcome and recurrence of ESCC and can be a novel predictor for poor prognosis of ESCC patients after curative resection.



Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150.
Day NE, Varghese C. Oesophageal cancer. Cancer Surv. 1994;19–20:43–54.
Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359–1365.
Vallbohmer D, Lenz HJ. Predictive and prognostic molecular markers in outcome of esophageal cancer. Dis Esophagus. 2006;19:425–432.
Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479.
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533.
Finley D, Ciechanover A, Varshavsky A. Ubiquitin as a central cellular regulator. Cell. 2004;116:S29–S32.
Sridhar VV, Kapoor A, Zhang K, Zhu J, et al. Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature. 2007;447:735–738.
Liu H, Buus R, Clague MJ, Urbe S. Regulation of ErbB2 receptor status by the proteasomal DUB POH1. PLoS ONE. 2009;4:e5544.
Singhal S, Taylor MC, Baker RT. Deubiquitylating enzymes and disease. BMC Biochem. 2008;9:S3.
Yao T, Song L, Xu W, DeMartino GN, et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol. 2006;8:994–1002.
Schreiner P, Chen X, Husnjak K, Randles L, et al. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature. 2008;453:548–552.
Husnjak K, Elsasser S, Zhang N, Chen X, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488.
Hamazaki J, Iemura S, Natsume T, Yashiroda H, et al. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 2006;25:4524–4536.
Qiu XB, Ouyang SY, Li CJ, Miao S, et al. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J. 2006;25:5742–5753.
Wicks SJ, Haros K, Maillard M, Song L, et al. The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-beta signalling. Oncogene. 2005;24:8080–8084.
Wicks SJ, Grocott T, Haros K, Maillard M, et al. Reversible ubiquitination regulates the Smad/TGF-beta signalling pathway. Biochem Soc Trans. 2006;34:761–763.
Cutts AJ, Soond SM, Powell S, Chantry A. Early phase TGFbeta receptor signalling dynamics stabilised by the deubiquitinase UCH37 promotes cell migratory responses. Int J Biochem Cell Biol. 2011;43:604–612.
Chen Z, Niu X, Li Z, Yu Y, et al. Effect of ubiquitin carboxy-terminal hydrolase 37 on apoptotic in A549 cells. Cell Biochem Funct. 2011;29:142–148.
Rolen U, Kobzeva V, Gasparjan N, Ovaa H, et al. Activity profiling of deubiquitinating enzymes in cervical carcinoma biopsies and cell lines. Mol Carcinog. 2006;45:260–269.
Fang Y, Mu J, Ma Y, Ma D, et al. The interaction between ubiquitin C-terminal hydrolase 37 and glucose-regulated protein 78 in hepatocellular carcinoma. Mol Cell Biochem. 2012;359:59–66.
Kapuria V, Peterson LF, Fang D, Bornmann WG, et al. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276.
Al-Shami A, Jhaver KG, Vogel P, Wilkins C, et al. Regulators of the proteasome pathway, Uch37 and Rpn13, play distinct roles in mouse development. PLoS ONE. 2010;5:e13654.
Fang Y, Fu D, Shen XZ. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806:1–6.
Acknowledgments
The authors would like to express gratitude to the staff of Prof. Xizhong Shen’s laboratory for their critical discussion and reading of the manuscript and the members of the Department of Thoracic Surgery of Zhongshan Hospital for their tumor specimens and follow-up data. This study was supported by the Shanghai Science and Technology Commission (10410709400, 10411950100), National Nature Science Foundation of China (81000968, 81101540, 81101637 and 81172273) and the National Clinical Key Special Subject of China.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yanjie Chen and Da Fu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Y., Fu, D., Xi, J. et al. Expression and Clinical Significance of UCH37 in Human Esophageal Squamous Cell Carcinoma. Dig Dis Sci 57, 2310–2317 (2012). https://doi.org/10.1007/s10620-012-2181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2181-9