Abstract
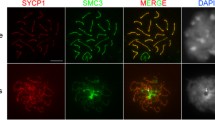
Special chromosomes limited to the germ line (=Ks) and exceptional genetic events such as elimination mitoses and a monopolar migration of the Ks in the last gonial mitosis are specific features of the complex chromosome cycle occurring in the chironomid Acricotopus lucidus. In the male, this unequal differential gonial mitosis results in a regular spermatocyte possessing all the Ks in addition to the somatic chromosomes (=Ss) and an aberrant spermatocyte containing only Ss. During evolution, the Ks have developed from the Ss and are composed of euchromatic S-homologous sections and heterochromatic segments. Less is known about the function and the transcriptional activity of the Ks. Specific post-translational histone modifications are known to be associated with transcriptionally active and inactive states of the chromatin. In an immunofluorescence study, the distribution of the following acetylated (ac), methylated (me) and phosphorylated (ph) amino acids in the histones H3 and H4 was analysed in Ks and Ss in male gonial mitoses and meiosis of A. lucidus, namely H3K18ac and H4K8ac, H3K4me3 and H3K9me3, H3S10ph, H3S28ph and H3T3ph. Ks and Ss clearly differ in the distribution of H3S28ph in gonial and meiotic metaphases. The H3S28ph mark covered the entire Ss, while the Ks showed this label only on their pericentromeric heterochromatin bands containing germ line-specific repetitive DNA sequences. A differential timing in the dephosphorylation of H3S10ph, H3S28ph and H3T3ph between Ks and Ss within the same cell was detected in the last gonial mitosis. The dephosphorylation occurred earlier in the Ks migrating first to the pole, than in the later equally segregating Ss. A programmed rapid histone deacetylation and dephosphorylation happened in the unseparated Ss of the aberrant spermatocyte at metaphase I in the connected primary spermatocyte, which correlated with the beginning of a permanent inactivation of these Ss in a metaphase-like condensed state. In meiosis, phosphorylated H3T3 could be detected only in metaphase II chromosomes at the inner centromeres of the attached sister chromatids. The H3T3ph labelling at this region was recently reported to be essential in mitosis for correct deposition of components of the chromosomal passenger complex and so for proper alignment, sister chromatid cohesion and segregation of chromosomes (Wang et al., Science 330:231–235, 2010; Curr Biol 21:1061–1069, 2011). Importantly, in spermatocytes, the euchromatic sections of the Ks were strongly acetylated at H3K18 and H4K8, and trimethylated at H3K4 during meiosis I and II, while the euchromatin of the meiotic Ss was hypoacetylated and hypomethylated at these sites. This result suggests a silencing of the Ss during spermatocyte meiosis. The high levels of active histone modifications detected in the euchromatic K sections support the idea that the Ks of A. lucidus are transcriptionally active in the germ line.









Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- FISH:
-
Fluorescence in situ hybridisation
- FITC:
-
Fluorescein isothiocyanate
- Ks:
-
Germ line-limited (‘Keimbahn’) chromosomes
- PBS:
-
Phosphate buffered saline
- Ss:
-
Somatic chromosomes
References
Adams RR, Maiato H, Earnshaw WC, Carmena M (2001) Essential roles of Drosophila inner centromere protein (INCENP) and Aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol 153:865–879
Banerjee T, Chakravarti D (2011) A peek into the complex realm of histone phosphorylation. Mol Cell Biol 31:4858–4873
Bannister AJ, Kouzarides T (2011) Regulations of chromatin by histone modifications. Cell Res 21:381–395
Bantock CR (1970) Experiments on the chromosome elimination in the gall midge, Mayetiola destructor. J Embryol Exp Morph 24:257–286
Bauer H, Beermann W (1952) Der Chromosomencyclus der Orthocladiinen (Nematocera, Diptera). Z Naturforsch 7:557–563
Bonenfant D, Towbin H, Coulot M, Schindler P, Mueller DR, Van Oostrum J (2007) Analysis of dynamic changes in post-translational modifications of human histones during cell cycle by mass spectrometry. Mol Cell Proteomics 6:1917–1932
Cabrero J, Teruel M, Carmona FD, Jimenez R, Camacho JPM (2007) Histone H3 lysine 9 acetylation pattern suggests that X and B chromosomes are silenced during entire male meiosis in a grasshopper. Cytogen Genome Res 119:135–142
Dai J, Sultan S, Taylor SS, Higgins JMG (2005) The kinase Haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev 19:472–488
Dai J, Sullivan BA, Higgins JMG (2006) Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell 11:41–750
Escriba MC, Giardini MC, Goday C (2011) Histone H3 phosphorylation and non-disjunction of the maternal X chromosome during male meiosis in sciarid flies. J Cell Sci 124:1715–1725
Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD (2005) Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438:1116–1122
Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM (2008) Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453:1132–1135
Gao S, Giansanti MG, Buttrick GJ, Ramasubramanyan S, Auton A, Gatti M, Wakefield JG (2008) Australin: a chromosomal passenger protein required specifically for Drosophila melanogaster male meiosis. J Cell Biol 180:521–535
Gerbi S (1986) Unusual movements in sciarid flies. In: Hennig W (ed) Germ line-soma differentiation. Results and problems of cell differentiation. Springer, New York, pp 71–104
Geyer-Duszynska I (1966) Genetic factors in oögenesis and spermatogenesis in Cecidomyiidae. In: Darlington CD, Lewis KR (eds) Chromosomes today, vol 1. Oliver and Boyd, Edinburgh, pp 174–178
Giet R, Glover DM (2001) Drosophila Aurora B kinase is required for histone H3 phosphorylation and Condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol 152:669–681
Goday C, Esteban MR (2001) Chromosome elimination in sciarid flies. BioEssays 23:242–250
Goto H, Yasui Y, Nigg EA, Inagaki M (2002) Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes Cells 7:11–17
Greciano PG, Ruiz MF, Kremer L, Goday C (2009) Two new chromodomain-containing proteins that associate with heterochromatin in Sciara coprophila chromosomes. Chromosoma 118:361–376
Grewal SIS, Jia S (2007) Heterochromatin revisted. Nat Genet 8:35–46
Hendzel MJ, Wie Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360
Hsu TC (1971) Heterochromatin pattern in metaphase chromosomes of Drosophila melanogaster. J Hered 62:285–287
Itoh Y, Kampf K, Pigozzi MI, Arnold AP (2009) Molecular cloning and characterization of the germline-restricted chromosome sequence in the zebra finch. Chromosoma 118:527–536
Jeppesen P (1997) Histone acetylation: a possible mechanism for the inheritance of cell memory at mitosis. BioEssays 19:67–74
Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H (2010) Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330:235–239
Kojima NF, Kojima KK, Kobayakawa S, Higashide N, Hamanaka C, Nitta A, Koeda I, Yamaguchi T, Shichiri M, Kohno S, Kubota S (2010) Whole chromosome elimination and chromosome terminus elimination both contribute to somatic differentiation in Taiwanese hagfish Paramyxine shen. Chromosome Res 18:383–400
Kunz W, Trepte H-H, Bier K (1970) On the function of the germ line chromosomes in the oogenesis of Wachtliella persicariae (Cecidomyiidae). Chromosoma 30:180–192
Kwon Y-G, Lee SY, Choi Y, Greengard P, Nairn AC (1997) Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc Natl Acad Sci USA 94:2168–2173
MacDonald VE, Howe LJ (2009) Histone acetylation. Epigenetics 4:139–143
Muramoto T, Müller I, Thomas G, Melvin A, Chubb JR (2010) Methylation of H3K4 is required for inheritance of active transcriptional states. Curr Biol 20:397–406
Ng HH, Robert F, Young RA, Struhl K (2003) Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell 11:709–719
Nowak SJ, Corces VG (2004) Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet 20:214–220
Pigozzi MI, Solari AJ (2005) The germ-line-restricted chromosome in the zebra finch: recombination in females and elimination in males. Chromosoma 114:403–409
Qian J, Lesage B, Beullens M, Van Eynde A, Bollen M (2011) PP1/Repo-Man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal Aurora B targeting. Curr Biol 21:766–773
Redi AA, Garagna S, Zacharias H, Zuccotti M, Capanna E (2001) The other chromatin. Chromosoma 110:136–147
Ruchaud S, Carmena M, Earnshaw WC (2007) Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol 8:798–812
Santos-Rosa H, Caldas C (2005) Chromatin modifier enzymes, the histone code and cancer. Eur J Cancer 41:2381–2402
Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T (2004) Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol 6:73–77
Schübeler D, MacAlpine DM, Scalzo D, Wirbelauer C, Kooperberg C, Van Leewen F, Gottschling DE, O’Neill LP, Turner BM, Delrow J, Bell SP, Groudine M (2004) The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev 18:1263–1271
Shilatifard A (2008) Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol 20:341–348
Staiber W (1988) G-banding of germ line limited chromosomes in Acricotopus lucidus (Diptera, Chironomidae). Chromosoma 97:231–234
Staiber W (2002) Isolation of a new germ line-specific repetitive DNA family in Acricotopus by microdissection of polytenized germ line limited chromosome sections from a permanent larval salivary gland preparation. Cytogenet Genome Res 98:210–215
Staiber W (2007) Asymmetric distribution of mitochondria and of spindle microtubules in opposite directions in differential mitosis of germ line cells in Acricotopus. Cell Tissue Res 329:197–203
Staiber W (2008) Centrosome hyperamplification with the formation of multiple asters and programmed chromosome inactivation in aberrant spermatocytes during male meiosis in Acricotopus. Cell Tissue Res 334:81–91
Staiber W (2009) FISH analysis and cytogenetic characterization of male meiotic prophase I in Acricotopus lucidus (Diptera, Chironomidae). Genet Mol Res 8:1218–1230
Staiber W, Schiffkowski C (2000) Structural evolution of the germ line-limited chromosomes in Acricotopus. Chromosoma 109:343–349
Staiber W, Thudium D (1986) X-ray induced rearrangements between germ-line limited and soma chromosomes of Acricotopus lucidus (Diptera, Chironomidae). Genetica 69:149–156
Staiber W, Wech I, Preiss A (1997) Isolation and localization of a germ line-specific highly repetitive DNA family in Acricotopus lucidus (Diptera, Chironomidae). Chromosoma 106:267–275
Su TT, Sprenger F, DiGregorio PJ, Campbell SD, O’Farrel PH (1998) Exit from mitosis in Drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Gene Dev 12:1495–1503
Valls E, Sanchez-Molina S, Martinez-Balbas MA (2005) Role of histone modifications in marking and activating genes through mitosis. J Biol Chem 280:42592–42600
Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K (2008) Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40:897–903
Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JMG (2010) Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330:231–235
Wang F, Ulanova NP, Van der Waal MS, Patnaik D, Lens SMA, Higgins JMG (2011) A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol 21:1061–1069
Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD (1998) Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA 95:7480–7484
White MJD (1973) Animal cytology and evolution, 3rd edn. Cambridge University Press, Cambridge, pp 500–546
Xu D, Bai J, Duan Q, Costa M, Dai W (2009) Covalent modifications of histones during mitosis and meiosis. Cell Cycle 8:3688–3694
Zakharova IS, Shevchenko AI, Shilov AG, Nesterova TB, Vandeberg JL, Zakian SM (2011) Histone H3 trimethylationat lysine 9 marks the inactive metaphase X chromosome in the marsupial Monodelphis domestica. Chromosoma 120:177–183
Zhao R, Nakamura T, Fu Y, Zsolt L, Spector DL (2011) Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat Cell Biol 13:1295–1303
Acknowledgments
The author thanks Clara Goday, CSIC, Madrid, Spain, for introducing in immunostaining and kindly providing antibodies; Sabrina Kugler, Anja C. Nagel and Prof. Anette Preiss, University of Hohenheim, for the gift of antibodies and support; and Prof. Neil Jones, Biological Sciences, Aberystwyth University, Wales, and the reviewers for helpful suggestions and corrections to the manuscript. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (Sta 462/5–1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Walther Traut.
Rights and permissions
About this article
Cite this article
Staiber, W. Germ line-limited and somatic chromosomes of Acricotopus lucidus differ in distribution and timing of alterations of histone modifications in male gonial mitosis and meiosis. Chromosome Res 20, 717–734 (2012). https://doi.org/10.1007/s10577-012-9308-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-012-9308-x