Abstract
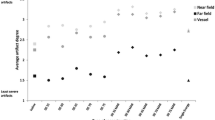
To compare the value of inversion recovery with on-resonant water suppression (IRON) to conventional T1-weighted (T1w) MRA and computed tomography angiography (CTA) for visualization of peripheral nitinol stents. We visualized 14 different peripheral nitinol stents in vitro both using Gadolinium (Gd) and ultrasmall superparamagnetic iron nanoparticles (USPIOs) for conventional T1w and IRON-MRA using clinical grade 1.5T MR scanner and iodinated contrast material for CTA using a 256-slice CT scanner. Parameter assessment included signal- and contrast-to-noise ratio (S/CNR), relative in-stent signal and artificial lumen narrowing. X-ray angiography served as gold standard for diameter assessment. Gd-enhanced IRON-MRA exhibited highest in-stent SNR and CNR values compared to conventional T1w MRA (IRON (Gd/USPIO): SNR = 30 ± 3/21 ± 2, CNR = 23 ± 2/14 ± 1; T1w: SNR = 16 ± 1/14 ± 2, CNR = 12 ± 1/10 ± 1, all p < 0.05). Furthermore, IRON-MRA achieved highest relative in-stent signal both using Gd and USPIO (IRON (Gd/USPIO): 121 ± 8 %/103 ± 6 %; T1w: 73 ± 2 %/66 ± 4 %; CTA: 84 ± 6 %, all p < 0.05). However, artificial lumen narrowing appeared similar in all imaging protocols (IRON (Gd/USPIO): 21 ± 3 %/21 ± 2 %; T1w: 16 ± 4 %/17 ± 3 %; CTA: 19 ± 2 %, all p = NS). Finally, IRON-MRA provided improvement of the in-stent lumen visualization with an ‘open-close-open’ design, which revealed a complete in-stent signal loss in T1w MRA. IRON-MRA improves in-stent visualization in vitro compared to conventional T1w MRA and CTA. In light of the in vitro results with Gd-enhanced IRON-MRA, the clinical implementation of such an approach appears promising.




Similar content being viewed by others
References
Fowkes FG, Rudan D, Rudan I et al (2013) Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382:1329–1340
Laird JR, Katzen BT, Scheinert D et al (2010) Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 3:267–276
Laird JR, Katzen BT, Scheinert D et al (2012) Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther 19:1–9
Layden J, Michaels J, Bermingham S, Higgins B, Guideline Development G (2012) Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 345:e4947
Norgren L, Hiatt WR, Dormandy JA et al (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45(Suppl S):S5–S67
Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH (2013) Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 268:719–728
Einstein AJ (2009) Radiation protection of patients undergoing cardiac computed tomographic angiography. JAMA 301:545–547
Health Quality O (2010) Stenting for peripheral artery disease of the lower extremities: an evidence-based analysis. Ont Health Technol Assess Ser 10:1–88
Brockmann C, Jochum S, Sadick M et al (2009) Dual-energy CT angiography in peripheral arterial occlusive disease. Cardiovasc Intervent Radiol 32:630–637
Edelman RR, Storey P, Dunkle E et al (2007) Gadolinium-enhanced off-resonance contrast angiography. Magn Reson Med 57:475–484
Frolich AM, Pilgram-Pastor SM, Psychogios MN, Mohr A, Knauth M (2011) Comparing different MR angiography strategies of carotid stents in a vascular flow model: toward stent-specific recommendations in MR follow-up. Neuroradiology 53:359–365
Bartels LW, Bakker CJ, Viergever MA (2002) Improved lumen visualization in metallic vascular implants by reducing RF artifacts. Magn Reson Med 47:171–180
Klemm T, Duda S, Machann J et al (2000) MR imaging in the presence of vascular stents: a systematic assessment of artifacts for various stent orientations, sequence types, and field strengths. J Magn Reson Imaging 12:606–615
Maintz D, Kugel H, Schellhammer F, Landwehr P (2001) In vitro evaluation of intravascular stent artifacts in three-dimensional MR angiography. Invest Radiol 36:218–224
Straube T, Wolf S, Flesser A et al (2005) MRI of carotid stents: influence of stent properties and sequence parameters on visualization of the carotid artery lumen. Rofo 177:375–380
Stuber M, Gilson WD, Schar M et al (2007) Positive contrast visualization of iron oxide-labeled stem cells using inversion-recovery with ON-resonant water suppression (IRON). Magn Reson Med 58:1072–1077
Korosoglou G, Shah S, Vonken EJ et al (2008) Off-resonance angiography: a new method to depict vessels–phantom and rabbit studies. Radiology 249:501–509
Vonken EJ, Korosoglou G, Yu J, Schar M, Weissleder R, Stuber M (2009) On the dual contrast enhancement mechanism in frequency-selective inversion-recovery magnetic resonance angiography (IRON-MRA). Magn Reson Med 62:314–324
Steen H, Andre F, Korosoglou G et al (2011) In vitro evaluation of 56 coronary artery stents by 256-slice multi-detector coronary CT. Eur J Radiol 80:143–150
Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ, Stuber M (2002) “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 48:658–666
Maintz D, Seifarth H, Raupach R et al (2006) 64-slice multidetector coronary CT angiography: in vitro evaluation of 68 different stents. Eur Radiol 16:818–826
Hahnel S, Trossbach M, Braun C et al (2003) Small-vessel stents for intracranial angioplasty: in vitro comparison of different stent designs and sizes by using CT angiography. AJNR 24:1512–1516
Wang Y, Truong TN, Yen C et al (2003) Quantitative evaluation of susceptibility and shielding effects of nitinol, platinum, cobalt-alloy, and stainless steel stents. Magn Reson Med 49:972–976
Gitsioudis G, Stuber M, Arend I et al (2013) Steady-state equilibrium phase inversion recovery ON-resonant water suppression (IRON) MR angiography in conjunction with superparamagnetic nanoparticles. A robust technique for imaging within a wide range of contrast agent dosages. J Magn Reson Imaging 38:836–844
Lettau M, Sauer A, Heiland S, Rohde S, Bendszus M, Hahnel S (2009) Carotid artery stents: in vitro comparison of different stent designs and sizes using CT angiography and contrast-enhanced MR angiography at 1.5 T and 3 T. AJNR 30:1993–1997
Lettau M, Sauer A, Heiland S et al (2010) In vitro comparison of different carotid artery stents: a pixel-by-pixel analysis using CT angiography and contrast-enhanced MR angiography at 1.5 and 3 T. Neuroradiology 52:823–830
Kuehne T, Saeed M, Moore P et al (2002) Influence of blood-pool contrast media on MR imaging and flow measurements in the presence of pulmonary arterial stents in swine. Radiology 223:439–445
Bunck AC, Juttner A, Kroger JR et al (2012) 4D phase contrast flow imaging for in-stent flow visualization and assessment of stent patency in peripheral vascular stents–a phantom study. Eur J Radiol 81:e929–e937
Hahnel S, Nguyen-Trong TH, Rohde S et al (2006) 3.0T contrast-enhanced MR angiography of carotid artery stents: in vitro measurements as compared with 1.5T. J Neuroradiol 33:75–80
European Stroke O, Tendera M, Aboyans V et al (2011) ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 32:2851–2906
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49:685–690
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR Images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Kromrey ML, Liedtke KR, Ittermann T et al (2016) Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol. doi:10.1007/s00330-016-4418-z
Reimer P, Tombach B (1998) Hepatic MRI with SPIO: detection and characterization of focal liver lesions. Eur Radiol 8:1198–1204
Hamm B, Staks T, Taupitz M et al (1994) Contrast-enhanced MR imaging of liver and spleen: first experience in humans with a new superparamagnetic iron oxide. J Magn Reson Imaging 4:659–668
Neuwelt EA, Hamilton BE, Varallyay CG et al (2009) Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int 75:465–474
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10554_2016_955_MOESM1_ESM.docx
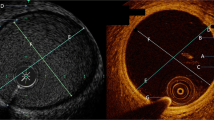
Supplementary material 1. Figure 1 Phantoms for the assessment of optimum concentration of contrast materials. Identical stepwise increase of Gadolinium (Gd) and ultrasmall superparamagnetic nanoparticles (USPIO, P904) contrast materials were used for all images A.–D.. Comparable SNR/CNR values revealed for 2µmol/ml of both Gd and USPIOs (circled sample). IRON-MRA provides increasing signal strength for higher concentrations of USPIO than T1-weighted MRA (B.–D.). Arrows in D. indicate signal-enhanced characteristic dipolar structures. MRA indicates magnetic resonance angiography; IRON, inversion recovery with on-resonant water suppression. Figure 2 Schematic illustration of the stent phantom (mash) placed in a box filled with normal saline solution. Regions of interest (ROIs, red circles) for assessment of signal intensity (SI) are placed in three in-stent segments (one middle and two lateral segments, SIIn−Stent,1−3), one ROI placed in an intraluminal non-stented tube segment (SITube), one ROI placed in the adjacent saline solution (SISaline) and one ROI placed in the background (SIBackground). (DOCX 669 KB)
Rights and permissions
About this article
Cite this article
Gitsioudis, G., Fortner, P., Stuber, M. et al. Off-resonance magnetic resonance angiography improves visualization of in-stent lumen in peripheral nitinol stents compared to conventional T1-weighted acquisitions: an in vitro comparison study. Int J Cardiovasc Imaging 32, 1645–1655 (2016). https://doi.org/10.1007/s10554-016-0955-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0955-4