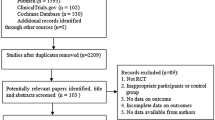
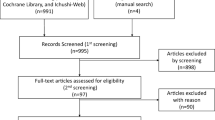
Abstract
Clinical trials conducted in Western countries have shown that aromatase inhibitors are associated with better disease-free survival (DFS) than tamoxifen in postmenopausal early breast cancer. Because pharmacogenetic differences in drug-metabolizing genes may cause ethnic differences, assessment of the efficacy and tolerability of aromatase inhibitors in non-white women is warranted. This open-label, randomized clinical trial included 706 postmenopausal Japanese women with hormone-receptor-positive breast cancer, who had received tamoxifen for 1 to 4 years as adjuvant therapy. This study was closed early after entry of ~28% of the initially planned patients. They were randomly assigned to either switch to anastrozole or to continue tamoxifen for total treatment duration of 5 years. Primary endpoints were DFS and adverse events. At a median follow-up of 42 months, the unadjusted hazard ratio was 0.69 (95% confidence interval, 0.42–1.14; P = 0.14) for DFS and 0.54 (95% CI, 0.29–1.02; P = 0.06) for relapse-free survival (RFS), both in favor of anastrozole. The incidence of thromboembolic events in the tamoxifen group and bone fractures in the anastrozole group was not excessively high. Switching from tamoxifen to anastrozole was likely to decrease disease recurrence in postmenopausal Japanese breast cancer patients. Ethnic differences in major adverse events may be attributable to a low baseline risk of these events in Japanese.




Similar content being viewed by others
References
The editorial board of the cancer statistics in Japan (2008) Cancer statistics in Japan, 2008. Foundation for promotion of cancer research, Tokyo, p 35
Berry DA, Cronin KA, Plevritis SK et al (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792
Fisher B, Costantino JP, Wickerham DL et al (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
The Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45–53
Coates AS, Keshaviah A, Thürlimann B et al (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25:486–492
Boccardo F, Rubagotti A, Puntoni M et al (2005) Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol 23:5138–5147
Jakesz R, Jonat W, Gnant M et al (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366:455–462
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Goetz MP, Rae JM, Suman VJ et al (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318
Broly F, Gaedigk A, Heim M et al (1991) Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol 10:545–558
Yokota H, Tamura S, Furuya H et al (1993) Evidence for a new variant CYP2D6 allele CYP2D6 J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics 3:256–263
Ma CX, Adjei AA, Salavaggione OE et al (2005) Human aromatase: gene resequencing and functional genomics. Cancer Res 65:11071–11082
Moy B, Tu D, Pater JL et al (2006) Clinical outcomes of ethnic minority women in MA.17: a trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer. Ann Oncol 17:1637–1643
Sobin LH, Fleming ID (1997) TNM classification of malignant tumors, 5th edn. Wiley, New York
Lakatos E, Lan KK (1992) A comparison of sample size methods for the logrank statistic. Stat Med 11:179–191
Boccardo F, Rubagotti A, Guglielmini P et al (2006) Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol 17(Suppl 7):vii 10–vii 14
Coombes RC, Kilburn LS, Snowdon CF et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study). Lancet 369:559–570
Barrett-Connor E, Siris ES, Wehren LE et al (2005) Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194
White RH, Zhou H, Romano PS (1998) Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med 128:737–740
Acknowledgments
We are indebted to all women who participated in the study. We thank all physicians contributing to this trial. We thank Mizuki Yamauchi and Yumiko Nomura for data management, and Hitoshi Masuda for his editorial assistance. This study was funded by Comprehensive Support Project for Oncology Research (CSPOR) of Public Health Research Foundation. The corporate and individual sponsors of this study are listed on the CSPOR website (http://www.csp.or.jp/cspor/kyousan_e.html). The pharmaceutical manufacturer/distributor who had provided financial contribution as a corporate sponsor took no part in this study other than providing information relevant to proper use of the study drugs. All decisions concerning the planning, implementation and publication of this study were made by the executive committee of this study.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Aihara, T., Takatsuka, Y., Ohsumi, S. et al. Phase III randomized adjuvant study of tamoxifen alone versus sequential tamoxifen and anastrozole in Japanese postmenopausal women with hormone-responsive breast cancer: N-SAS BC03 study. Breast Cancer Res Treat 121, 379–387 (2010). https://doi.org/10.1007/s10549-010-0888-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0888-x