Abstract
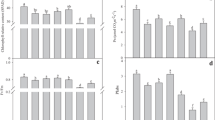
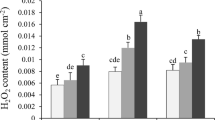
Changes in chlorophyll content, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) binding protein (RBP), Rubisco activase (RA), Rubisco large (LS) and small (SS) subunits, and electrolyte leakage were investigated in wheat leaf segments during heat stress (HS) for 1 h and for 24 h at 40 °C in darkness or in light, as well as after recovery from heat stress (HSR) for 24 h at 25 °C in light. The 24-h HS treatment in darkness decreased irreversibly photosynthetic pigments, soluble proteins, RBP, RA, Rubisco LS and SS. An increase in RA and RBP protein contents was observed under 24-h HS and HSR in light. This increase was in accordance with their role as chaperones and the function of RBP as a heat shock protein.
Similar content being viewed by others
Abbreviations
- EC:
-
electrical conductivity
- f.m.:
-
fresh mass
- HS:
-
heat stress
- HSP:
-
heat shock proteins
- HSR:
-
recovery after heat stress
- RA:
-
Rubisco activase
- RBP:
-
Rubisco binding protein
- Rubisco LS:
-
Rubisco large subunit
- Rubisco SS:
-
Rubisco small subunit
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase
References
Ben-Zvi, A.P., Goloubinoff, P.: Mechanisms of disaggregation and refolding of stable protein aggregation by molecular chaperones.-J. Struct. Biol. 135: 84–93, 2001.
Bose, A., Tiwari, B.S., Chattopadhyay, M.K., Gupta, S., Ghosh, B.: Thermal stress induces differences degradation of Rubisco in heat-sensitive and heat-tolerant rice-Physiol. Plant. 105: 89–94, 1999.
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding-Anal. Biochem. 72: 248–254, 1976.
Crafts-Brandner, S.J., Van de Loo, F.J., Salvucci, M.E.: The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature.-Plant Physiol. 114: 439–444, 1997.
Demirevska-Kepova, K., Juperlieva-Mateeva, B.: Purification of Rubisco large subunit binding protein from barley and preparation of polyclonal antisera against it.-Compt. rend. Acad. Sci. Bulg. 43: 101–104, 1990.
Demirevska-Kepova, K., Simova, L.: Isolation and purification of ribulose-1,5-bisphosphate carboxylase/oxygenase from barley leaves.-Bulg. J. Plant Physiol. 15: 3–10, 1989.
Ferguson, D.L., Al-Khatib, K., Guikema, J.A., Paulsen, G.M.: Degradation of proteins from thylakoid membranes in senescencing wheat leaves at high temperature.-Plant Cell Environ. 16: 421–428, 1993.
Georgieva, K.: Some mechanisms of damage and acclimation of the photosynthetic apparatus to high temperature.-Bulg. J. Plant Physiol. 25: 89–99, 1999.
Grover, A., Sabat, S.C., Mohanty, P.: Effect of temperature on photosynthetic activities of senescing detached wheat leaves.-Plant Cell Physiol. 27: 117–126, 1986.
Harding, S. A., Guikema, J.A., Paulsen, G.M.: Photosynthetic decline from high temperature stress during maturation of wheat. I. Interaction with senescence processes.-Plant Physiol. 92: 648–653, 1990.
Hartl, F.-U., Hlodan, R., Langer, T.: Molecular chaperones in protein folding: the art of avoiding stick situations.-Trends biol. Sci. 19: 20–25, 1994.
Hildbrand, M., Fisher, A., Feller, U.: Protein catabolism in bean leaf discs: Accumulation of a soluble fragment of ribulose-1,5-bisphosphate carboxylase/oxygenase under oxygen deficiency.-J. exp. Bot. 45: 1197–1204, 1994.
Hortensteiner, S., Feller, U.: Nitrogen metabolism and remobilization during senescence.-J. exp. Bot. 53: 927–937, 2002.
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the heat of bacteriophage T4.-Nature 227: 680–685, 1970.
Law, R., Crafts-Brander, S.: Inhibition of photosynthesis to heat stress is closely correlated with activation of Rubisco.-Plant Physiol. 120: 173–181, 1999.
Law, R.D., Crafts-Brander, S.J., Salvucci, M.E.: Heat stress induces the synthesis of a new form of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in cotton leaves.-Planta 214: 117–125, 2001.
Ledesma, N.A., Kawabata, S., Sugiama, N.: Effect of high temperature on protein expression in strawberry plants.-Biol. Plant. 48: 73–79, 2004.
Mitsuhashi, W., Feller, U.: Effects of light and external solutes on the catabolism of nuclear-encoded stromal proteins in intact chloroplasts isolated from pea leaves.-Plant Physiol. 100: 2100–2105, 1992.
Morales, D., Rodrigues, P., Dell’Amico, J., Nicolas, E., Torrecillas, A., Sanchez-Blanco, M.J.: High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato.-Biol. Plant. 47: 203–208, 2003/4.
Musgrove, J.E., Jonson, R.A., Ellis, R.J.: Dissociation of the ribulose bisphosphate carboxylase large subunit binding protein into dissimilar subunits.-Eur. J. Biochem. 163: 529–534, 1987.
Okada, K., Inoue, Y., Satoh, K., Katoh, S.: Effects of light on degradation of chlorophyll and proteins during senescence of detached rice leaves.-Plant Cell Physiol. 33: 1183–1191, 1992.
Portis, A.R., Jr.: Rubisco activase-Rubisco’s catalytic chaperone.-Photosynth. Res. 75: 11–27, 2003.
Salvucci, M.E., Osteryoung, K.W., Crafts-Brander, S.J., Vierling, E.: Exceptional sensitivity of rubisco activase to thermal denaturation in vitro and in vivo.-Plant Physiol. 127: 1053–1064, 2001.
Salvucci, M.E., Portis, A.R., Ogren, W.L.: A soluble chloroplast protein catalyses ribulosebisphosphate carboxylase/oxygenase activation in vivo.-Photosynth. Res. 7: 193–201, 1985.
Sanches de Jimenes, E., Medrano L., Martinez-Barajas, E. Rubisco activase, a possible new member of the molecular chaperone family.-Biochemistry 34: 2826–2831 1995.
Schmitz, G., Schmidt, M., Feierabend, J.: Comparision of the expression of a plastid chaperonin 60 in different plant tissue and under photosynthetic and non photosynthetic conditions.-Planta 200: 326–334, 1996.
Strain, H.H., Cope, B.T., Svec, W.A.: Analytical procedures for the isolation, identification, estimation and investigation of the chlorophylls.-Methods Enzymol. 23: 452–476, 1971.
Todorov, D.T., Karanov, E.N., Smith, A.R., Hall, M.A.: Chlorophyllase activity and chlorophyll content in wild type and eti 5 mutant of Arabidopsis thaliana subjected to low and high temperatures.-Biol. Plant. 46: 633–636, 2003.
Weis, E.: The temperature sensitivity of dark-inactivation and light-inactivation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts.-FEBS Lett. 129: 197–200, 1981.
Xu, Q., Paulsen, A.Q., Guikema, J.A., Paulsen, G.M.: Functional and ultrastructural injury to photosynthesis in wheat by high temperature during maturation.-Environ. exp. Bot. 35: 43–54, 1995.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was partially supported by Swiss National Science Foundation (Project 31-55289.98).
Rights and permissions
About this article
Cite this article
Demirevska-Kepova, K., Holzer, R., Simova-Stoilova, L. et al. Heat stress effects on ribulose-1,5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol Plant 49, 521–525 (2005). https://doi.org/10.1007/s10535-005-0045-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10535-005-0045-2