Abstract
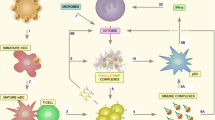
Systemic lupus erythematosus (SLE) is a complex disease resulting from inflammatory responses of the immune system against several autoantigens. Inflammation is conditioned by the continuous presence of autoantibodies and leaked autoantigens, e.g. from not properly cleared dying and dead cells. Various soluble molecules and biophysical properties of the surface of apoptotic cells play significant roles in the appropriate recognition and further processing of dying and dead cells. We exemplarily discuss how Milk fat globule epidermal growth factor 8 (MFG-E8), biophysical membrane alterations, High mobility group box 1 (HMGB1), C-reactive protein (CRP), and anti-nuclear autoantibodies may contribute to the etiopathogenesis of the disease. Up to date knowledge about these key elements may provide new insights that lead to the development of new treatment strategies of the disease.







Similar content being viewed by others
References
Lorenz HM, Grunke M, Hieronymus T, Herrmann M, Kuhnel A, Manger B, Kalden JR (1997) In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum 40:306–317
Dillon SR, Constantinescu A, Schlissel MS (2001) Annexin V binds to positively selected B cells. J Immunol 166:58–71
Appelt U, Sheriff A, Gaipl US, Kalden JR, Voll RE, Herrmann M (2005) Viable, apoptotic and necrotic monocytes expose phosphatidylserine: cooperative binding of the ligand Annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell Death Differ 12:194–196
Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304:1147–1150
Gaipl US, Kuenkele S, Voll RE, Beyer TD, Kolowos W, Heyder P, Kalden JR, Herrmann M (2001) Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ 8:327–334
Mevorach D, Mascarenhas JO, Gershov D, Elkon KB (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med 188:2313–2320
Gaipl US, Beyer TD, Heyder P, Kuenkele S, Bottcher A, Voll RE, Kalden JR, Herrmann M (2004) Cooperation between C1q and DNase I in the clearance of necrotic cell-derived chromatin. Arthritis Rheum 50:640–649
Lood C, Gullstrand B, Truedsson L, Olin AI, Alm GV, Ronnblom L, Sturfelt G, Eloranta ML, Bengtsson AA (2009) C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum 60:3081–3090
Oshima K, Aoki N, Kato T, Kitajima K, Matsuda T (2002) Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur J Biochem 269:1209–1218
Aoki N, Ishii T, Ohira S, Yamaguchi Y, Negi M, Adachi T, Nakamura R, Matsuda T (1997) Stage specific expression of milk fat globule membrane glycoproteins in mouse mammary gland: comparison of MFG-E8, butyrophilin, and CD36 with a major milk protein, beta-casein. Biochim Biophys Acta 1334:182–190
Nakatani H, Aoki N, Nakagawa Y, Jin-No S, Aoyama K, Oshima K, Ohira S, Sato C, Nadano D, Matsuda T (2006) Weaning-induced expression of a milk-fat globule protein, MFG-E8, in mouse mammary glands, as demonstrated by the analyses of its mRNA, protein and phosphatidylserine-binding activity. Biochem J 395:21–30
Oshima K, Aoki N, Negi M, Kishi M, Kitajima K, Matsuda T (1999) Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem Biophys Res Commun 254:522–528
Burgess BL, Abrams TA, Nagata S, Hall MO (2006) MFG-E8 in the retina and retinal pigment epithelium of rat and mouse. Mol Vis 12:1437–1447
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187
Shi J, Gilbert GE (2003) Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood 101:2628–2636
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Miyasaka K, Hanayama R, Tanaka M, Nagata S (2004) Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur J Immunol 34:1414–1422
Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, Huber C, Kosco-Vilbois MH, Zinkernagel R, Miele G, Aguzzi A (2008) Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med 205:1293–1302
Hanayama R, Tanaka M, Miwa K, Nagata S (2004) Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol 172:3876–3882
Hanayama R, Nagata S (2005) Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc Natl Acad Sci USA 102:16886–16891
Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M (2004) Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med 200:459–467
Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M (2002) Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum 46:191–201
Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH (2005) The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA 102:9258–9263
Couto JR, Taylor MR, Godwin SG, Ceriani RL, Peterson JA (1996) Cloning and sequence analysis of human breast epithelial antigen BA46 reveals an RGD cell adhesion sequence presented on an epidermal growth factor-like domain. DNA Cell Biol 15:281–286
Yamaguchi H, Takagi J, Miyamae T, Yokota S, Fujimoto T, Nakamura S, Ohshima S, Naka T, Nagata S (2008) Milk fat globule EGF factor 8 in the serum of human patients of systemic lupus erythematosus. J Leukoc Biol 83:1300–1307
Hu CY, Wu CS, Tsai HF, Chang SK, Tsai WI, Hsu PN (2009) Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus 18:676–681
Franz S, Frey B, Sheriff A, Gaipl US, Beer A, Voll RE, Kalden JR, Herrmann M (2006) Lectins detect changes of the glycosylation status of plasma membrane constituents during late apoptosis. Cytometry A 69:230–239
Mierke CT, Kollmannsberger P, Zitterbart DP, Smith J, Fabry B, Goldmann WH (2008) Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J 94:661–670
Mierke CT, Zitterbart DP, Kollmannsberger P, Raupach C, Schlotzer-Schrehardt U, Goecke TW, Behrens J, Fabry B (2008) Breakdown of the endothelial barrier function in tumor cell transmigration. Biophys J 94:2832–2846
Sheriff A, Gaipl US, Franz S, Heyder P, Voll RE, Kalden JR, Herrmann M (2004) Loss of GM1 surface expression precedes annexin V-phycoerythrin binding of neutrophils undergoing spontaneous apoptosis during in vitro aging. Cytometry A 62:75–80
Raupach C, Zitterbart DP, Mierke CT, Metzner C, Muller FA, Fabry B (2007) Stress fluctuations and motion of cytoskeletal-bound markers. Phys Rev E Stat Nonlin Soft Matter Phys 76:011918
Sessa L, Bianchi ME (2007) The evolution of High Mobility Group Box (HMGB) chromatin proteins in multicellular animals. Gene 387:133–140
Thomas JO, Travers AA (2001) HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci 26:167–174
Tsuda K, Kikuchi M, Mori K, Waga S, Yoshida M (1988) Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry 27:6159–6163
Bianchi ME, Agresti A (2005) HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev 15:496–506
Melvin VS, Edwards DP (1999) Coregulatory proteins in steroid hormone receptor action: the role of chromatin high mobility group proteins HMG-1 and -2. Steroids 64:576–586
Melvin VS, Roemer SC, Churchill ME, Edwards DP (2002) The C-terminal extension (CTE) of the nuclear hormone receptor DNA binding domain determines interactions and functional response to the HMGB-1/-2 co-regulatory proteins. J Biol Chem 277:25115–25124
Jayaraman L, Moorthy NC, Murthy KG, Manley JL, Bustin M, Prives C (1998) High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev 12:462–472
Agresti A, Lupo R, Bianchi ME, Muller S (2003) HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun 302:421–426
Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S, Bianchi ME (1999) The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 22:276–280
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3:995–1001
Chen G, Li J, Ochani M, Rendon-Mitchell B, Qiang X, Susarla S, Ulloa L, Yang H, Fan S, Goyert SM, Wang P, Tracey KJ, Sama AE, Wang H (2004) Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol 76:994–1001
Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P (2007) The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81:84–91
Semino C, Angelini G, Poggi A, Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106:609–616
Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP (2006) HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol 290:C990–C999
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Bell CW, Jiang W, Reich CF III, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291:C1318–C1325
Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE (2008) Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 205:3007–3018
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5:825–830
Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P (2005) Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol 35:2184–2190
Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496
Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110:1970–1981
Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99–103
Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS (2008) High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol 180:5067–5074
Sha Y, Zmijewski J, Xu Z, Abraham E (2008) HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180:2531–2537
Bianchi ME, Manfredi AA (2007) High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220:35–46
Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA (2008) Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29:21–32
Chen GY, Tang J, Zheng P, Liu Y (2009) CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323:1722–1725
Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R, Chiorazzi N, Tracey KJ, Andersson U, Harris HE (2002) High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum 46:2598–2603
Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I (2003) High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum 48:971–981
Popovic K, Ek M, Espinosa A, Padyukov L, Harris HE, Wahren-Herlenius M, Nyberg F (2005) Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum 52:3639–3645
Barkauskaite V, Ek M, Popovic K, Harris HE, Wahren-Herlenius M, Nyberg F (2007) Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus 16:794–802
Fadok VA, McDonald PP, Bratton DL, Henson PM (1998) Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans 26:653–656
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I (1997) Immunosuppressive effects of apoptotic cells. Nature 390:350–351
Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR (1998) Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum 41:1241–1250
Hayashi A, Nagafuchi H, Ito I, Hirota K, Yoshida M, Ozaki S (2009) Lupus antibodies to the HMGB1 chromosomal protein: epitope mapping and association with disease activity. Mod Rheumatol 19:283–292
Uesugi H, Ozaki S, Sobajima J, Osakada F, Shirakawa H, Yoshida M, Nakao K (1998) Prevalence and characterization of novel pANCA, antibodies to the high mobility group non-histone chromosomal proteins HMG1 and HMG2, in systemic rheumatic diseases. J Rheumatol 25:703–709
Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE (2007) Factors masking HMGB1 in human serum and plasma. J Leukoc Biol 81:67–74
Kushner I (1988) The acute phase response: an overview. Methods Enzymol 163:373–383
Baumann H, Gauldie J (1994) The acute phase response. Immunol Today 15:74–80
Zhang D, Jiang SL, Rzewnicki D, Samols D, Kushner I (1995) The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem J 310(Pt 1):143–148
Kleemann R, Gervois PP, Verschuren L, Staels B, Princen HM, Kooistra T (2003) Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-beta complex formation. Blood 101:545–551
Taylor AW, Ku NO, Mortensen RF (1990) Regulation of cytokine-induced human C-reactive protein production by transforming growth factor-beta. J Immunol 145:2507–2513
Szalai AJ, Wu J, Lange EM, McCrory MA, Langefeld CD, Williams A, Zakharkin SO, George V, Allison DB, Cooper GS, Xie F, Fan Z, Edberg JC, Kimberly RP (2005) Single-nucleotide polymorphisms in the C-reactive protein (CRP) gene promoter that affect transcription factor binding, alter transcriptional activity, and associate with differences in baseline serum CRP level. J Mol Med 83:440–447
Garlanda C, Bottazzi B, Bastone A, Mantovani A (2005) Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 23:337–366
Du Clos TW, Mold C (2004) C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res 30:261–277
Motie M, Brockmeier S, Potempa LA (1996) Binding of model soluble immune complexes to modified C-reactive protein. J Immunol 156:4435–4441
Potempa LA, Siegel JN, Fiedel BA, Potempa RT, Gewurz H (1987) Expression, detection and assay of a neoantigen (Neo-CRP) associated with a free, human C-reactive protein subunit. Mol Immunol 24:531–541
Wang HW, Sui SF (2001) Dissociation and subunit rearrangement of membrane-bound human C-reactive proteins. Biochem Biophys Res Commun 288:75–79
Diehl EE, Haines GK 3rd, Radosevich JA, Potempa LA (2000) Immunohistochemical localization of modified C-reactive protein antigen in normal vascular tissue. Am J Med Sci 319:79–83
Heuertz RM, Schneider GP, Potempa LA, Webster RO (2005) Native and modified C-reactive protein bind different receptors on human neutrophils. Int J Biochem Cell Biol 37:320–335
Devaraj S, Du Clos TW, Jialal I (2005) Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects. Arterioscler Thromb Vasc Biol 25:1359–1363
Dobrinich R, Spagnuolo PJ (1991) Binding of C-reactive protein to human neutrophils. Inhibition of respiratory burst activity. Arthritis Rheum 34:1031–1038
Zouki C, Haas B, Chan JS, Potempa LA, Filep JG (2001) Loss of pentameric symmetry of C-reactive protein is associated with promotion of neutrophil-endothelial cell adhesion. J Immunol 167:5355–5361
Potempa LA, Zeller JM, Fiedel BA, Kinoshita CM, Gewurz H (1988) Stimulation of human neutrophils, monocytes, and platelets by modified C-reactive protein (CRP) expressing a neoantigenic specificity. Inflammation 12:391–405
Volanakis JE (2001) Human C-reactive protein: expression, structure, and function. Mol Immunol 38:189–197
Hack CE, Wolbink GJ, Schalkwijk C, Speijer H, Hermens WT, van den Bosch H (1997) A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol Today 18:111–115
Pepys MB, Booth SE, Tennent GA, Butler PJ, Williams DG (1994) Binding of pentraxins to different nuclear structures: C-reactive protein binds to small nuclear ribonucleoprotein particles, serum amyloid P component binds to chromatin and nucleoli. Clin Exp Immunol 97:152–157
Robey FA, Jones KD, Steinberg AD (1985) C-reactive protein mediates the solubilization of nuclear DNA by complement in vitro. J Exp Med 161:1344–1356
Du Clos TW (1989) C-reactive protein reacts with the U1 small nuclear ribonucleoprotein. J Immunol 143:2553–2559
Sheriff A, Gaipl US, Voll RE, Kalden JR, Herrmann M (2004) Apoptosis and systemic lupus erythematosus. Rheum Dis Clin North Am 30:505–527 viii-ix
Li SH, Szmitko PE, Weisel RD, Wang CH, Fedak PW, Li RK, Mickle DA, Verma S (2004) C-reactive protein upregulates complement-inhibitory factors in endothelial cells. Circulation 109:833–836
Gershov D, Kim S, Brot N, Elkon KB (2000) C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med 192:1353–1364
Marnell L, Mold C, Du Clos TW (2005) C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol 117:104–111
Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB (1999) C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med 190:1733–1740
Janko C, Schorn C, Weidner D, Sarter K, Chaurio R, Sheriff A, Schett G, Munoz LE (2009) Treatment with DNAse I fosters binding to nec PBMC of CRP. Autoimmunity 42:286–288
Van Molle W, Denecker G, Rodriguez I, Brouckaert P, Vandenabeele P, Libert C (1999) Activation of caspases in lethal experimental hepatitis and prevention by acute phase proteins. J Immunol 163:5235–5241
Szalai AJ (2004) C-reactive protein (CRP) and autoimmune disease: facts and conjectures. Clin Dev Immunol 11:221–226
Nakayama S, Du Clos TW, Gewurz H, Mold C (1984) Inhibition of antibody responses to phosphocholine by C-reactive protein. J Immunol 132:1336–1340
Xia D, Samols D (1997) Transgenic mice expressing rabbit C-reactive protein are resistant to endotoxemia. Proc Natl Acad Sci USA 94:2575–2580
Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW (2002) C-reactive protein mediates protection from lipopolysaccharide through interactions with Fc gamma R. J Immunol 169:7019–7025
Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, Cobb AJ, Ley SV, Aquilina JA, Robinson CV, Sharif I, Gray GA, Sabin CA, Jenvey MC, Kolstoe SE, Thompson D, Wood SP (2006) Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440:1217–1221
ter Borg EJ, Horst G, Limburg PC, van Rijswijk MH, Kallenberg CG (1990) C-reactive protein levels during disease exacerbations and infections in systemic lupus erythematosus: a prospective longitudinal study. J Rheumatol 17:1642–1648
Pepys MB, Lanham JG, De Beer FC (1982) C-reactive protein in SLE. Clin Rheum Dis 8:91–103
Russell AI, Cunninghame Graham DS, Shepherd C, Roberton CA, Whittaker J, Meeks J, Powell RJ, Isenberg DA, Walport MJ, Vyse TJ (2004) Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet 13:137–147
Das T, Sen AK, Kempf T, Pramanik SR, Mandal C (2003) Induction of glycosylation in human C-reactive protein under different pathological conditions. Biochem J 373:345–355
Rordorf C, Schnebli HP, Baltz ML, Tennent GA, Pepys MB (1982) The acute-phase response in (NZB X NZW)F1 and MRL/l MICE. J Exp Med 156:1268–1273
Ogden CA, Elkon KB (2005) Single-dose therapy for lupus nephritis: C-reactive protein, nature’s own dual scavenger and immunosuppressant. Arthritis Rheum 52:378–381
Rodriguez W, Mold C, Kataranovski M, Hutt J, Marnell LL, Du Clos TW (2005) Reversal of ongoing proteinuria in autoimmune mice by treatment with C-reactive protein. Arthritis Rheum 52:642–650
Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, Marion TN, Volanakis JE (2003) Delayed lupus onset in (NZB x NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum 48:1602–1611
Du Clos TW, Zlock LT, Hicks PS, Mold C (1994) Decreased autoantibody levels and enhanced survival of (NZB × NZW) F1 mice treated with C-reactive protein. Clin Immunol Immunopathol 70:22–27
Bell SA, Faust H, Schmid A, Meurer M (1998) Autoantibodies to C-reactive protein (CRP) and other acute-phase proteins in systemic autoimmune diseases. Clin Exp Immunol 113:327–332
Sjowall C, Eriksson P, Almer S, Skogh T (2002) Autoantibodies to C-reactive protein is a common finding in SLE, but not in primary Sjogren’s syndrome, rheumatoid arthritis or inflammatory bowel disease. J Autoimmun 19:155–160
Rosenau BJ, Schur PH (2006) Antibodies to C reactive protein. Ann Rheum Dis 65:674–676
Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, Buyon JP (2006) Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest 116:2413–2422
Sjowall C, Bengtsson AA, Sturfelt G, Skogh T (2004) Serum levels of autoantibodies against monomeric C-reactive protein are correlated with disease activity in systemic lupus erythematosus. Arthritis Res Ther 6:R87–R94
Dieker JW, van der Vlag J, Berden JH (2002) Triggers for anti-chromatin autoantibody production in SLE. Lupus 11:856–864
Munoz LE, van Bavel C, Franz S, Berden J, Herrmann M, van der Vlag J (2008) Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus 17:371–375
Radic M, Marion T, Monestier M (2004) Nucleosomes are exposed at the cell surface in apoptosis. J Immunol 172:6692–6700
Casciola-Rosen LA, Anhalt G, Rosen A (1994) Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 179:1317–1330
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Solomon DH, Kavanaugh AJ, Schur PH (2002) Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 47:434–444
Hahn BH (1998) Antibodies to DNA. N Engl J Med 338:1359–1368
Swaak T, Smeenk R (1985) Detection of anti-dsDNA as a diagnostic tool: a prospective study in 441 non-systemic lupus erythematosus patients with anti-dsDNA antibody (anti-dsDNA). Ann Rheum Dis 44:245–251
Linnik MD, Hu JZ, Heilbrunn KR, Strand V, Hurley FL, Joh T (2005) Relationship between anti-double-stranded DNA antibodies and exacerbation of renal disease in patients with systemic lupus erythematosus. Arthritis Rheum 52:1129–1137
Winkler TH, Jahn S, Kalden JR (1991) IgG human monoclonal anti-DNA autoantibodies from patients with systemic lupus erythematosus. Clin Exp Immunol 85:379–385
Winkler TH, Fehr H, Kalden JR (1992) Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol 22:1719–1728
Herrmann M, Winkler TH, Fehr H, Kalden JR (1995) Preferential recognition of specific DNA motifs by anti-double-stranded DNA autoantibodies. Eur J Immunol 25:1897–1904
Koffler D, Schur PH, Kunkel HG (1967) Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med 126:607–624
Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A (2007) Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology (Oxford) 46:1052–1056
Raz E, Brezis M, Rosenmann E, Eilat D (1989) Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol 142:3076–3082
Ehrenstein MR, Katz DR, Griffiths MH, Papadaki L, Winkler TH, Kalden JR, Isenberg DA (1995) Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int 48:705–711
Mjelle JE, Kalaaji M, Rekvig OP (2009) Exposure of chromatin and not high affinity for dsDNA determines the nephritogenic impact of anti-dsDNA antibodies in (NZB × NZW)F1 mice. Autoimmunity 42:104–111
Ravirajan CT, Rowse L, MacGowan JR, Isenberg DA (2001) An analysis of clinical disease activity and nephritis-associated serum autoantibody profiles in patients with systemic lupus erythematosus: a cross-sectional study. Rheumatology (Oxford) 40:1405–1412
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349:1526–1533
ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG (1990) Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum 33:634–643
Mackworth-Young CG, Chan JK, Bunn CC, Hughes GR, Gharavi AE (1986) Complement fixation by anti-dsDNA antibodies in SLE: measurement by radioimmunoassay and relationship with disease activity. Ann Rheum Dis 45:314–318
Kramers C, Hylkema MN, van Bruggen MC, van de Lagemaat R, Dijkman HB, Assmann KJ, Smeenk RJ, Berden JH (1994) Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest 94:568–577
Zhao Z, Weinstein E, Tuzova M, Davidson A, Mundel P, Marambio P, Putterman C (2005) Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum 52:522–530
Jacob CO, Zang S, Li L, Ciobanu V, Quismorio F, Mizutani A, Satoh M, Koss M (2003) Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice. J Immunol 171:1564–1571
Berkel AI, Petry F, Sanal O, Tinaztepe K, Ersoy F, Bakkaloglu A, Loos M (1997) Development of systemic lupus erythematosus in a patient with selective complete C1q deficiency. Eur J Pediatr 156:113–115
Mevorach D (2000) Opsonization of apoptotic cells. Implications for uptake and autoimmunity. Ann N Y Acad Sci 926:226–235
Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ (1998) Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies [see comments]. Nat Genet 19:56–59
Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP (1992) Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol 2:1345–1354
Zack DJ, Stempniak M, Wong AL, Taylor C, Weisbart RH (1996) Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J Immunol 157:2082–2088
Rumore PM, Steinman CR (1990) Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J Clin Invest 86:69–74
Munoz LE, Janko C, Grossmayer GE, Frey B, Voll RE, Kern P, Kalden JR, Schett G, Fietkau R, Herrmann M, Gaipl US (2009) Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum 60:1733–1742
Frisoni L, McPhie L, Colonna L, Sriram U, Monestier M, Gallucci S, Caricchio R (2005) Nuclear autoantigen translocation and autoantibody opsonization lead to increased dendritic cell phagocytosis and presentation of nuclear antigens: a novel pathogenic pathway for autoimmunity? J Immunol 175:2692–2701
Grossmayer GE, Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, Herrmann M (2005) Removal of dying cells and systemic lupus erythematosus. Mod Rheumatol 15:383–390
Sarmiento LF, Munoz LE, Chirinos P, Bianco NE, Zabaleta-Lanz ME (2007) Opsonization by anti-dsDNA antibodies of apoptotic cells in systemic lupus erythematosus. Autoimmunity 40:337–339
Grazia Cappiello M, Sutterwala FS, Trinchieri G, Mosser DM, Ma X (2001) Suppression of Il-12 transcription in macrophages following Fc gamma receptor ligation. J Immunol 166:4498–4506
Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50:1861–1872
Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S (2006) Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443:998–1002
Acknowledgments
This work was supported by the “Deutsche Forschungsgemeinschaft” (HE 4490/3-1 and SFB 643-project B5), by the Training Grant SFB GK 643, by the Interdisciplinary Center for Clinical Research (IZKF) (N2) at the University Hospital of the Friedrich-Alexander University, by an intramural grant (ELAN-fonds M3-09.03.18.1) of the Medical Faculty of the Friedrich-Alexander University and by the K. und R. Wucherpfennigstiftung.
Author information
Authors and Affiliations
Corresponding author
Additional information
Luis E. Muñoz and Martin Herrmann equally contributed as senior authors.
Rights and permissions
About this article
Cite this article
Kruse, K., Janko, C., Urbonaviciute, V. et al. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis 15, 1098–1113 (2010). https://doi.org/10.1007/s10495-010-0478-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0478-8