Abstract
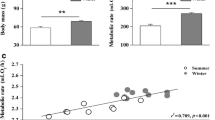
Changes in flight muscle size are important mediators of phenotypic flexibility in birds, so the ability to track such changes over time in individual birds is a valuable tool for investigating phenotypic flexibility. Ultrasonography has been used to track changes in flight muscle size in shorebirds, but has not been previously used to track such changes in small birds, despite variation in flight muscle size being an important contributor to phenotypic flexibility in these birds. One prominent avian example of phenotypic flexibility is the seasonal phenotypes of small birds in response to climatic variation. The winter phenotype in these birds is characterized by increases in organismal metabolic rates and pectoralis muscle mass. We measured seasonal flight muscle size in House Sparrows (Passer domesticus, 25–30 g) using both ultrasonography and wet muscle mass and tested the correlation between ultrasonographic measures of breast muscle thickness and muscle mass. We further tested whether ultrasonographic measures of muscle thickness were sufficiently precise to detect seasonal variation in flight muscle mass. Muscle mass was significantly and positively associated with ultrasonographic measurements of breast muscle thickness for short-axis (SA), long-axis (LA), and combined SA and LA measurements. Breast muscle mass was significantly greater in winter than in summer (17.5 %) and muscle thickness also increased significantly in winter for both SA (9.1 %) and LA (7.5 %) measures. Thus, these data confirm that winter elevations of flight muscle mass consistently contribute to the winter phenotype in House Sparrows and that ultrasonography is effective in detecting seasonal changes in muscle mass in small birds.
Zusammenfassung
Jahreszeitliche phänotypische Flexibilität in der Größe der Flugmuskulatur bei kleinen Vögeln: Ein Vergleich von Maßen anhand von Ultraschall und Gewebegewicht
Veränderungen der Flugmuskulatur von Vögeln sind wichtige Mediatoren phänotypischer Flexibilität. Von daher ist die Möglichkeit, derartige zeitliche Veränderungen in individuellen Vögeln zu messen ein sehr hilfreich um phänotypische Flexibilität zu untersuchen. Mittels Ultraschalls können Veränderungen in der Flugmuskulatur von Seevögeln gemessen werden. Diese Methode wurde jedoch bis jetzt noch nicht in Singvögeln genutzt, und das obwohl Variation in der Größe von Flugmuskeln in diesen Arten ein wichtiger Indikator von phänotypischer Flexibilität ist. Ein bekanntes Beispiel von phänotypischer Flexibilität in der Ornithologie sind die saisonalen Veränderungen von Phänotypen kleiner Vögel als Reaktion auf Klimavariation. Die winterlichen Phänotypen dieser Vögel sind durch einen Anstieg physiologischer Umsatzraten und des Gewichtes des Pectoralis Muskels geprägt. Wir haben die saisonale Größe der Flugmuskulatur in Haussperlingen (Passer domesticus, 25–30 g) mittels Ultraschalls sowie durch Bestimmung der Dicke und des Nassgewichtes, sowie die Korrelation zwischen beiden bestimmt. Ferner haben wir untersucht ob die Ultraschallmessung der Dicke der Muskeln die saisonale Veränderung in der Masse der Flugmuskeln vorhersagen kann. Muskelmasse war signifikant positiv mit den Ultraschallmaßen short-axis (SA), long-axis (LA) und einer Kombination von SA und LA assoziiert. Die Masse des Brustmuskels war im Winter signifikant höher als im Sommer (17.5 %). Ebenso nahm die Dicke der Muskeln während des Winters zu (SA: 9.1 %, LS: 7.5 %). Unsere Daten unterstützen dass die Zunahme der Flugmuskulatur während des Winters konsistent zum Winterphänotyp von Haussperlingen beitragen, und ferner, dass Ultraschall ausreichend effektiv ist um saisonale Veränderungen in der Muskelmasse kleiner Vögel zu detektieren.



Similar content being viewed by others
References
Arens JR, Cooper SJ (2005) Metabolic and ventilatory acclimatization to cold stress in house sparrows (Passer domesticus). Physiol Biochem Zool 78:579–589
Baggott GK (1975) Moult, flight muscle “hypertrophy” and premigratory lipid deposition of the juvenile Willow warbler, Phylloscopus trochilus. J Zool 175:299–314
Bairlein F (1995) Manual of field methods ESF (http://www.ifv-vogelwarte.de/ESF/manual.pdf)
Bauchinger U, McWilliams SR, Kolb H, Popenko VM, Price ER, Biebach H (2011) Flight muscle shape reliably predicts flight muscle mass of migratory songbirds: a new tool for field ornithologists. J Ornithol 152:507–514
Carrascal LM, Senar JC, Mozetich I, Uribe F, Domènech J (1998) Interactions among environmental stress, body condition, nutritional status, and dominance in great tits. Auk 115:727–738
Constantini D, Cardinale M, Carere C (2007) Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comp Biochem Physiol C 144:363–371
Cooper SJ (2002) Seasonal metabolic acclimatization in mountain chickadees and juniper titmice. Physiol Biochem Zool 75:386–395
Dawson WR, O’Connor TP (1996) Energetic features of avian thermoregulatory responses. In: Carey C (ed) Avian energetics and nutritional ecology. Chapman & Hall, New York, pp 85–124
Dietz MW, Dekinga A, Piersma T, Verhulst S (1999) Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiol Biochem Zool 72:28–37
Engstrand SM, Bryant DM (2002) A trade-off between clutch size and incubation efficiency in the barn swallow Hirundo rustica. Funct Ecol 16:782–791
Evans PR, Davidson NC, Uttley JD, Evans RD (1992) Premigratory hypertrophy of flight muscles: an ultrastructural study. Ornis Scand 23:238–243
Gosler AG, Harper DGC (2000) Assessing the heritability of body condition in birds: a challenge exemplified by the great tit, Parus major L. (Aves). Biol J Linn Soc 71:103–117
Guglielmo CG, Williams TD (2003) Phenotypic flexibility of body composition in relation to migratory state, age and sex in the western sandpiper. Physiol Biochem Zool 76:84–98
Hart JS (1962) Seasonal acclimatization in four species of small wild birds. Physiol Zool 35:224–236
Hohtola E (1982) Thermal and electromyographic correlates of shivering thermogenesis in the pigeon. Comp Biochem Physiol 73A:159–166
Köver G, Romvari R, Horn P, Berenyi E, Jensen JF, Sørensen P (1998) In vivo assessment of breast muscle, abdominal fat and total fat volume in meat-type chickens by magnetic resonance imaging. Acta Vet Hungar 46:135–144
Landys-Ciannelli MM, Piersma T, Jukema J (2003) Strategic size changes of internal organs and muscle tissue in the Bar-tailed Godwit during fat storage on a spring stopover site. Funct Ecol 17:151–159
Lessels CM, Boag PT (1987) Unreapeatable repeatabilities: a common mistake. Auk 104:116–121
Liknes ET, Swanson DL (2011) Phenotypic flexibility of body composition associated with seasonal acclimatization of passerine birds. J Therm Biol 36:363–370
Lindström Å, Kvist A, Piersma T, Dekinga A, Dietz M (2000) Avian pectoral muscle size rapidly tracks body mass changes during flight, fasting and fuelling. J Exp Biol 203:913–919
Marjoniemi K, Hohtola E (1999) Shivering thermogenesis in leg and breast muscles of Galliform chicks and nestlings of the domestic pigeon. Physiol Biochem Zool 72:484–492
Marsh RL (1984) Adaptations of the Gray Catbird Dumetella carolinensis to long-distance migration: flight muscle hypertrophy associated with elevated body mass. Physiol Zool 57:105–117
Marsh RL, Dawson WR (1989) Avian adjustments to cold. In: Wang LCH (ed) Advances in comparative and environmental physiology 4: animal adaptation to cold. Springer, New York, pp 205–253
McKechnie AE, Swanson DL (2010) Sources and significance of variation in basal, summit and maximal metabolic rates in birds. Curr Zool 56:741–758
Newton SF (1993) Body condition of a small passerine bird: ultrasonic assessment and significance in overwinter survival. J Zool Lond 229:561–580
O’Connor TP (1995) Metabolic characteristics and body composition in house finches: effects of seasonal acclimatization. J Comp Physiol B 165:298–305
Piersma T, Dietz MW (2007) Twofold seasonal variation in the supposedly constant, species-specific ratio of upstroke to downstroke flight muscles in red knots Calidris canutus. J Avian Biol 38:536–540
Piersma T, van Gils JA (2011) The flexible phenotype: a body-centred integration of ecology, physiology, and behavior. Oxford University Press, Oxford
Piersma T, Gudmundsson GA, Lilliendahl K (1999) Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Physiol Biochem Zool 72:405–415
Potti J, Moreno J, Merino S, Frias O, Rodriquez R (1999) Environmental and genetic variation in the haematocrit of fledgling pied flycatchers Ficedula hypoleuca. Oecologia 120:1–8
Pyle P (1997) Identification guide to North American birds. Slate Creek Press, Bolinas, California, USA, Part I. Columbidae to Ploceidae
Rae LR, Mitchell GW, Mauck RA, Guglielmo CG, Norris DR (2009) Radio transmitters do not affect the body condition of savannah sparrows during the fall premigratory period. J Field Ornithol 80:419–426
Senar JC, Conroy MJ, Carrascal LM, Domènech J, Mozetich I, Uribe F (1999) Identifying sources of heterogeneity in capture probabilities: an example using the Great Tit Parus major. Bird Study 46(suppl.):S248–S252
Senar JC, Domènech J, Uribe F (2002) Great Tits (Parus major) reduce body mass in response to wing area reduction: a field experiment. Behav Ecol 13:725-727
Swanson DL (1991) Substrate metabolism under cold stress in seasonally acclimatized dark-eyed juncos. Physiol Zool 64:1578–1592
Swanson DL (2001) Are summit metabolism and thermogenic endurance correlated in winter acclimatized passerine birds? J Comp Physiol B 171:475–481
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Bio. 209:466–474
Tallman DT, Swanson DL, Palmer JS (2002) Birds of South Dakota, 3rd edn. South Dakota Ornithologists’ Union, Aberdeen
Vézina F, Williams TD (2003) Plasticity in body composition in breeding birds: what drives the metabolic costs of egg production? Physiol Biochem Zool 76:716–773
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2007) Thermogenic side effects to migratory disposition in shorebirds. Am J Physiol Regul Integr Comp Physiol 292:1287–1297
Vézina F, Dekinga A, Piersma T (2011) Shorebirds’ seasonal adjustments in thermogenic capacity are reflected by changes in body mass: how preprogrammed and instantaneous acclimation work together. Integr Comp Biol 51:394–408
Ward S (1996) Energy expenditure of female barn swallows Hirundo rustica during egg formation. Physiol Zool 69:930–951
Winkler DW, Allen PE (1995) Effects of handicapping on female condition and reproduction in tree swallows (Tachycineta bicolor). Auk 112:737–747
Winkler DW, Allen PE (1996) The seasonal decline in tree swallow clutch size: physiological constraint or strategic adjustment? Ecology 77:922–932
Acknowledgments
We thank Amy Stephenson for help with conducting the ultrasound measurements. We also thank Greg Mitchell and an anonymous reviewer for their helpful comments on an earlier version of this manuscript. David Swanson was supported by NSF IOS 1021218.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Rights and permissions
About this article
Cite this article
Swanson, D.L., Merkord, C. Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements. J Ornithol 154, 119–127 (2013). https://doi.org/10.1007/s10336-012-0877-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0877-4