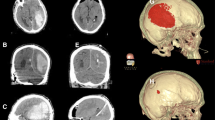
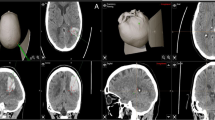
Abstract
Hematoma expansion is correlated with morbidity and mortality for patients with intracerebral hemorrhage (ICH). Recent studies demonstrated that contrast extravasation on contrast-enhanced CT and small-enhancing foci, so-called spot signs, on CT angiography are associated with subsequent hematoma enlargement. Such radiological markers of ICH may have significant implications not only as a surrogate marker for hematoma expansion in medical hemostatic therapy but also as indication for surgery. In this article, a brief description of contrast extravasation and “spot sign” will be provided first. The findings of some of the important trials that shaped the current landscape of therapeutic interventions for ICH will then be reviewed. Many neurosurgeons have faced a significant dilemma since the Surgical Trial in Intracerebral Haemorrhage (STICH) trial was published. Under adverse circumstances, many neurosurgeons assume that minimally invasive surgical interventions are still likely to benefit some patients and will be more effective. Among future candidate strategies for ICH, the most promising is neuroendoscopic surgery with direct hemostatic devices, which attains direct local hemostasis at the sites of vascular rupture. It is plausible that ultra-early direct hemostatic surgery given in the emergency setting might reduce hematoma volume and rebleeding and improve outcome. Finally, a description of future avenues of minimally invasive surgery for ICH treatment and suggestions for the design of further studies using reliable predictor of hematoma expansion spot sign will be provided. Neuroendoscopic interventions are minimally invasive and are likely of benefit in hemostasis and hematoma removal. On the basis of these observations, the spot sign of ICH has sub-emergency surgical implications.
Similar content being viewed by others
References
Adeoye O, Broderick JP (2010) Advances in the management of intracerebral hemorrhage. Nat Rev Neurol 6:593–601
Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G, Holzer P, Bone G, Mokry M, Korner E et al (1989) Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg 70:530–535
Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA (1999) Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 30:2025–2032
Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J (1997) Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28:1–5
Carhuapoma JR, Wang PY, Beauchamp NJ, Keyl PM, Hanley DF, Barker PB (2000) Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral hemorrhage. Stroke 31:726–732
Chakraborty S, Stotts G, Rush C, Hogan MJ, Dowlatshahi D (2011) Dynamic ‘spot sign’ resolution following INR correction in a patient with warfarin-associated intracerebral hemorrhage. Case Rep Neurol 3:154–159
Chen CC, Cho DY, Chang CS, Chen JT, Lee WY, Lee HC (2005) A stainless steel sheath for endoscopic surgery and its application in surgical evacuation of putaminal haemorrhage. J Clin Neurosci 12:937–940
Cheung RT (2007) Update on medical and surgical management of intracerebral hemorrhage. Rev Recent Clin Trials 2:174–181
Cho DY, Chen CC, Chang CS, Lee WY, Tso M (2006) Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol 65:547–555
Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66:1175–1181
Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, Oleinik A, Lev MH, Gonzalez RG, Romero JM (2009) Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke 40:2994–3000
Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, Goldstein JN, Rosand J, Lev MH, Gonzalez RG, Romero JM (2010) The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke 41:54–60
Delgado Almandoz JE, Kelly HR, Schaefer PW, Brouwers HB, Yoo AJ, Stone MJ, Goldstein JN, Rosand J, Lev MH, Gonzalez RG, Romero JM (2012) CT angiography spot sign predicts in-hospital mortality in patients with secondary intracerebral hemorrhage. J Neurointerv Surg 4(6):442–447
Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE (2011) Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 76:1238–1244
Ederies A, Demchuk A, Chia T, Gladstone DJ, Dowlatshahi D, Bendavit G, Wong K, Symons SP, Aviv RI (2009) Postcontrast CT extravasation is associated with hematoma expansion in CTA spot negative patients. Stroke 40:1672–1676
Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R (1998) Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 29:1160–1166
Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, Lev MH, Rosand J (2007) Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 68:889–894
Goldstein JN, Gilson AJ (2011) Critical care management of acute intracerebral hemorrhage. Curr Treat Options Neurol 13:204–216
Hallevi H, Abraham AT, Barreto AD, Grotta JC, Savitz SI (2010) The spot sign in intracerebral hemorrhage: the importance of looking for contrast extravasation. Cerebrovasc Dis 29:217–220
Hayashi N, Nishimura S, Numagami Y, Murakimi K, Inoue T, Obara H, Nishijima M (2006) Retrospective analysis of effects and complications in cases treated with endoscopic evacuation of intracerebral hemorrhage. No Shinkei Geka 34:1233–1238
Hemphill JC 3rd, Morabito D, Farrant M, Manley GT (2005) Brain tissue oxygen monitoring in intracerebral hemorrhage. Neurocrit Care 3:260–270
Hsieh PC, Cho DY, Lee WY, Chen JT (2005) Endoscopic evacuation of putaminal hemorrhage: how to improve the efficiency of hematoma evacuation. Surg Neurol 64:147–153
Juttler E, Steiner T (2007) Treatment and prevention of spontaneous intracerebral hemorrhage: comparison of EUSI and AHA/ASA recommendations. Expert Rev Neurother 7:1401–1416
Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T (1996) Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 27:1783–1787
Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T (1997) Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke 28:2370–2375
Kim J, Smith A, Hemphill JC 3rd, Smith WS, Lu Y, Dillon WP, Wintermark M (2008) Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol 29:520–525
Kirkman MA, Mahattanakul W, Gregson BA, Mendelow AD (2008) The effect of the results of the STICH trial on the management of spontaneous supratentorial intracerebral haemorrhage in Newcastle. Br J Neurosurg 22:739–746
Kuo LT, Chen CM, Li CH, Tsai JC, Chiu HC, Liu LC, Tu YK, Huang AP (2011) Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage: case selection, surgical technique, and long-term results. Neurosurg Focus 30:E9
Leira R, Davalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, Castillo J (2004) Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 63:461–467
Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T (2005) Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 352:777–785
Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T (2008) Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 358:2127–2137
Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH (2005) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 365:387–397
Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D (2008) Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl 105:147–151
Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC (2001) Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology 56:1294–1299
Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ (2010) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 41:2108–2129
Murai Y, Ikeda Y, Teramoto A, Tsuji Y (1998) Magnetic resonance imaging-documented extravasation as an indicator of acute hypertensive intracerebral hemorrhage. J Neurosurg 88:650–655
Murai Y, Takagi R, Ikeda Y, Yamamoto Y, Teramoto A (1999) Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg 91:424–431
Nagasaka T, Inao S, Ikeda H, Tsugeno M, Okamoto T (2008) Inflation–deflation method for endoscopic evacuation of intracerebral haematoma. Acta Neurochir (Wien) 150:685–690
Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Takagawa Y, Inao S, Wakabayashi T (2009) Balanced irrigation-suction technique with a multifunctional suction cannula and its application for intraoperative hemorrhage in endoscopic evacuation of intracerebral hematomas: technical note. Neurosurgery 65:E826–E827
Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T (2011) A novel monoshaft bipolar cautery for use in endoscopic intracranial surgery. A short technical note. Clin Neurol Neurosurg 113:607–611
Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T (2011) Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis 20:208–213
Nakano T, Ohkuma H, Ebina K, Suzuki S (2003) Neuroendoscopic surgery for intracerebral haemorrhage—comparison with traditional therapies. Minim Invasive Neurosurg 46:278–283
Nishihara T, Teraoka A, Morita A, Ueki K, Takai K, Kirino T (2000) A transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas. Technical note. J Neurosurg 92:1053–1055
Nishihara T, Nagata K, Tanaka S, Suzuki Y, Izumi M, Mochizuki Y, Akabane A, Ochiai C (2005) Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care 2:67–74
Orakcioglu B, Becker K, Sakowitz OW, Herweh C, Kohrmann M, Huttner HB, Steiner T, Unterberg A, Schellinger PD (2008) MRI of the perihemorrhagic zone in a rat ICH model: effect of hematoma evacuation. Neurocrit Care 8:448–455
Orakcioglu B, Becker K, Sakowitz OW, Unterberg A, Schellinger PD (2008) Serial diffusion and perfusion MRI analysis of the perihemorrhagic zone in a rat ICH model. Acta Neurochir Suppl 103:15–18
Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, Guterman LR, Hopkins LN (2003) Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med 31:1482–1489
Rincon F, Mayer SA (2010) Intracerebral hemorrhage: getting ready for effective treatments. Curr Opin Neurol 23:59–64
Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G (2003) Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke 34:968–974
Teernstra OP, Evers SM, Kessels AH (2006) Meta analyses in treatment of spontaneous supratentorial intracerebral haematoma. Acta Neurochir (Wien) 148:521–528
Vespa P, McArthur D, Miller C, O’Phelan K, Frazee J, Kidwell C, Saver J, Starkman S, Martin N (2005) Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care 2:274–281
Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, Symons SP (2007) CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 38:1257–1262
Wang WZ, Jiang B, Liu HM, Li D, Lu CZ, Zhao YD, Sander JW (2009) Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke 4:11–16
Yamamoto T, Nakao Y, Mori K, Maeda M (2006) Endoscopic hematoma evacuation for hypertensive cerebellar hemorrhage. Minim Invasive Neurosurg 49:173–178
Yamamoto T, Nakao Y, Osada H, Mori K, Maeda M (2006) Endoscopic hematoma evacuation for intracerebral hemorrhage. Jpn J Neurosurg (Tokyo) 16:533–540
Zuo Y, Cheng G, Gao DK, Zhang X, Zhen HN, Zhang W, Xiao SC (2009) Gross-total hematoma removal of hypertensive basal ganglia hemorrhages: a long-term follow-up. J Neurol Sci 287:100–104
Acknowledgments
Drs Yoichi Uozumi, Kyozo Kato, and Berk Orakcioglu provided important advice and support regarding this article. This article was supported by a JFE (The Japanese Foundation for Research and Promotion of Endoscopy) Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Atos Alves de Sousa, Belo Horizonte, Brazil
The surgical management of intracerebral hemorrhage is one of the most controversial subjects in neurosurgery. From the classical microsurgical approach to hematomas to the most recent methods, so-called minimally invasive, such as puncture–aspiration guided by stereotactic or neuronavigation, the endoscopic aspiration and coagulation of the bleeding point have always been attempted at. The use of drugs to lyse the clot and/or drugs to stop the bleeding has also been tried by several authors. The problem is that every trial proposed to validate the suggested method of treatment has failed to prove its effectiveness.
According to the literature, the “spot sign” seems to be a useful method to identify patients at risk for hematoma enlargement. The authors, after a very complete revision of the subject, propose to use the spot sign to stratify patients for surgical evacuation of the hematoma using endoscope and, at the same time, hemostasis of the bleeding points. However, as stated by them, further studies are needed to prove the efficacy of such method.
Hussam Metwali, Hannover, Germany
In this review article, the authors tried to answer the question “What does the CT angiography ‘spot sign’ of intracerebral hemorrhage mean in modern neurosurgical settings with minimally invasive endoscopic techniques?” The focus of the article is supposed to be the significance and the applications of the so-called spot sign but the authors went beyond this focus describing and comparing different techniques; meanwhile, the main focus of the article was relatively poor.
The authors discussed the “spot sign” as an indication for early intervention for intracerebral hematomas. However, there are other critical clinical and radiological indications for early intervention which the authors did not mention.
The authors used high temper in clarifying the advantages of the minimally invasive option for the management of intracerebral hematoma. It would have been better if the author used only statistics and numbers especially that neuroendoscopy and puncture are still in evaluation.
Louis J. Kim, Seattle, USA
This is a well-written and brief overview of the current state of ICH management and relevant past and present clinical studies. While the authors do not attempt to solve the complex issues of ICH patient management with their own clinical data or meta-analysis, they do succinctly summarize the main medical and surgical methodologies available.
This is a useful update to the readership of the current “state of the affairs” in ICH care, knowing that many neurosurgeons do not closely follow the critical care, stroke, and neurology literature where several of the studies summarized in this manuscript typically appear. Spot sign in the setting of ICH is a clear harbinger of worse outcome; however, the management of such cases is still controversial.The ongoing clinical trials alluded to in this article will help shape future management recommendations.
H. Bart Brouwers, USA, and Luca Regli, Switzerland
Intracerebral hemorrhage (ICH) is the most lethal form of stroke, with a one-month mortality of approximately 40 percent [1]. To date, no treatment for ICH has shown benefit in a phase III randomized controlled trial. Location and volume of the initial hemorrhage are the most important determinants of outcome following ICH, but are non-modifiable once patients present to the emergency department [2,3]. Hematoma expansion, the third prominent predictor of outcome [4,5], is potentially preventable and is therefore a common target in clinical trials, including both medical and surgical interventions [6-8].
The CT angiography (CTA) spot sign was first described in 1999 by Becker et al. and its definition was refined in 2007 [9-11]. Over the last five years, numerous, mostly retrospective, studies have shown the spot sign to be independently associated with both hematoma expansion and poor outcome [10-12] The recently published PREDICT study confirmed this association in a prospective fashion, targeting patients presenting within six hours from symptom onset [13]. Furthermore, a recent study found that the association between spot sign and hematoma expansion is independent of time from symptom onset, therefore providing a predictive marker for hematoma expansion in all ICH patients [14]. This highlights the potential of the spot sign to not only have diagnostic and prognostic value, but to also become a tool for treatment stratification. It opens a path for trial design with guided interventions based on neuroimaging. Two ongoing trials are already using the spot sign to select patients for treatment with recombinant factor VIIa (STOP-IT [ClinicalTrials.gov NCT00810888] and SPOTLIGHT [ClinicalTrials.gov NCT01359202]). Another trial, SCORE-IT, aims to look at the role of the spot sign in guiding intensive blood pressure reduction [15].
The aforementioned trials focus on the medical management of ICH patients, leaving the main question of the provocative review in this issue of Neurosurgical Review by Nagasaka et al. unaddressed: what is the role of the CTA spot sign in neurosurgery [16]? To answer this question, two points must be considered: 1.) The biological and pathophysiological underpinnings of the CTA spot sign and 2.) The current evidence for (neurosurgical) interventions in acute ICH.
Although the biology behind the spot sign is not yet fully understood, various studies have shed light on potential mechanisms. First, several studies show anticoagulation to be strongly associated with spot sign presence [17, 18]. Second, a recent genetic association study found that the apolipoprotein E ε2 allele is associated with both spot sign presence and hematoma expansion [18]. Third, a model proposed by Dr. Fisher in 1971 incorporates the first two points in his ‘avalanche model’ of hematoma expansion, where the initial hematoma expands and ruptures adjacent diseased vessels surrounding the hematoma, causing additional bleeding, extravasation of contrast (i.e. spot sign), and hematoma expansion [19].
Regarding the status of interventions for acute ICH, we are currently awaiting the results of two potentially game changing clinical trials: INTERACT2 [6] and STICH II [8], both of which are expected to be presented during the 2013 European Stroke Conference. The first evaluates aggressive blood pressure reduction to prevent hematoma expansion, while the latter is investigating the surgical evacuation of lobar hematomas within one centimeter of the cortical surface without intraventricular extension [6, 8]. Although the original STICH trial did not show a benefit of surgery in unselected ICH patients [20], a Cochrane review and a recent patient-level meta-analysis demonstrated a positive effect of surgical evacuation in certain subgroups, raising hope that STICH II will be a positive trial [21, 22]. If this is indeed the case, the spot sign and its underlying biology should be considered when making treatment decisions based on CTA findings. In these patients, the dilemma has been and will continue to be: to operate or not to operate? Given the worse prognosis of patients with a spot sign and their highly increased risk of hematoma expansion, surgical intervention seems to be justified in some cases. In addition, we have learned from the PREDICT trial and other studies that spot sign positive patients have larger ICH volumes at baseline [13]. This provides further rationale for decompression of the hematoma, given the strong relationship between ICH volume and outcome [3]. However, bearing in mind the underlying biology of potentially altered coagulation status plus diseased small vessels, all neurosurgeons must ensure meticulous hemostasis before finishing the procedure. This is somewhat similar to the dilemma whether or not to operate on a patient with cerebral amyloid angiopathy. Also, the clinical signs of rebleeding should be monitored closely, as mentioned by Nagasaka et al [16]. Although the community awaits definite answers and practical guidelines, the role of the CTA spot sign appears to be both diagnostic and clinically meaningful in the neurosurgical setting.
References
1. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176
2. Flaherty ML, Haverbusch M, Sekar P, Kissela B, Kleindorfer D, Moomaw CJ, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66:1182–1186
3. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993
4. Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181
5. Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244
6. Delcourt C, Huang Y, Wang J, Heeley E, Lindley R, Stapf C, et al. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2). Int J Stroke. 2010;5:110–116
7. Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15:559–576
8. Mendelow AD, Gregson BA, Mitchell PM, Murray GD, Rowan EN, Gholkar AR, et al. Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials. 2011;12:124
9. Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032
10. Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894
11. Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography "spot sign" predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262
12. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of inhospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60
13. Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–314
14. Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, Schwab K, et al. CTA Spot Sign Predicts Hematoma Expansion in Patients with Delayed Presentation After Intracerebral Hemorrhage. Neurocrit Care. 2012 Aug 10. [Epub ahead of print]
15. Goldstein JN, Brouwers HB, Romero JM, McNamara KA, Schwab K, Greenberg SM, et al. SCORE-IT: The Spot Sign Score in Restricting ICH Growth - An ATACH-II Ancillary Study. J Vasc Interv Neurol. 2012;5:20–25
16. Nagasaka T, Inao S, Wakabayashi T. What does the CT angiography “spot sign” of intracerebral hemorrhage mean in modern neurosurgical settings with minimally invasive endoscopic techniques? Neurosurg Rev. 2012
17. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000
18. Brouwers HB, Biffi A, McNamara KA, Ayres AM, Valant V, Schwab K, et al. Apolipoprotein E Genotype Is Associated With CT Angiography Spot Sign in Lobar Intracerebral Hemorrhage. Stroke. 2012;43:2120–2125
19. Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–550
20. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397
21. Prasad K, Mendelow AD, Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev. 2008;(4):CD000200
22. Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, et al. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43:1496–1504
Sources of Funding
Dr. Brouwers was supported by the National Institutes of Health – National Institute of Neurological Disorders and Stroke (NIH – NINDS) SPOTRIAS fellowship grant P50NS051343. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the NINDS.
Rights and permissions
About this article
Cite this article
Nagasaka, T., Inao, S. & Wakabayashi, T. What does the CT angiography “spot sign” of intracerebral hemorrhage mean in modern neurosurgical settings with minimally invasive endoscopic techniques?. Neurosurg Rev 36, 341–348 (2013). https://doi.org/10.1007/s10143-012-0437-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-012-0437-7