Abstract
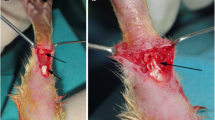
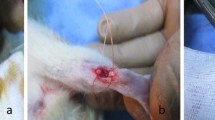
Diabetes mellitus (DM) is associated with musculoskeletal damage. Investigations have indicated that healing of the surgically tenotomized Achilles tendon was considerably augmented following low-level laser therapy (LLLT) in non-diabetic, healthy animals. The aim of the present study was to evaluate the effect of LLLT on the Achilles tendon healing in streptozotocin-induced diabetic (STZ-D) rats via a biomechanical evaluating method. Thirty-three rats were divided into non-diabetic (n = 18) and diabetic (n = 15) groups. DM was induced in the rats by injections of STZ. The right Achilles tendons of all rats were tenotomized 1 month after STZ injections. The two experimental groups (n = 6 for each group) of non-diabetic rats were irradiated with a helium–neon (He–Ne) laser at 2.9 and 11.5 J/cm2 for ten consecutive days. The two experimental groups of diabetic rats (n = 5 for each group) were irradiated with a He–Ne laser at 2.9 and 4.3 J/cm2 for ten consecutive days. The tendons were submitted to a tensiometric test. Significant improvements in the maximum stress (MS) values (Newton per square millimeter) were found following LLLT at 2.9 J/cm2 in both the non-diabetic (p = 0.031) and diabetic (p = 0.019) experimental groups when compared with their control groups. LLLT at 2.9 J/cm2 to the tenotomized Achilles tendons in the non-diabetic and diabetic rats significantly increased the strength and MS of repairing Achilles tendons in our study.



Similar content being viewed by others
References
Kemmis K (2010) Common musculoskeletal disorders in older adults with diabetes. Top Geriatr Rehabil 26:264–272
Perkins I (2004) Diabetes mellitus epidemiology—classification, determinants and public health impacts. J Miss State Med Assoc 45:355–362
Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8:29–41
Ramchurn N, Mashamba C, Leitch E, Arutchelvam V, Narayanan K, Weaver J, Hamilton J, Heycock C, Saravanan V, Kelly C (2009) Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med 20:718–721
Cagliero E, Apruzzese W, Perlimutter G, Nathan DM (2002) Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am J Med 20:718–721
Chaturvedi N (2007) The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract 76(suppl 1):S3–S12
Marks RM (2001) Complications of foot and ankle surgery in patients with diabetes. Clin Orthop Relat Res 391:153–161
Ganesh SP, Pietrobon R, Cecilio WA, Pan D, Lightdale N, Nunley JA (2005) The impact of diabetes on patient outcomes after ankle fracture. J Bone Joint Surg Am 87:1712–1718
Akturk M, Karaahmetoglu S, Kacar M, Muftuoglu O (2002) Thickness of the supraspinatous and biceps tendons in diabetic patients. Diabet Care 25:408
Smith LL, Burnet SP, Mc Neil JD (2003) Musculoskeletal manifestations of diabetes mellitus. Br J Sports Med 37:30–35
Lin TW, Gardenas L, Soslowsky LJ (2004) Biomechanics of tendon injury and repair. J Biochem 37:865–877
Mavrikakis ME, Drimis S, Kontoyannis DA, Rasidakis A, Moulolpoulou ES, Kontoyannis S (1989) Calcific shoulder periarthritis (tendinitis) in adult onset diabetes mellitus: a controlled study. Ann Rheum Dis 48:211–214
Akturk M, Ozdemir A, Maral I, Yektin I, Arslan M (2007) Evaluation of Achilles tendon thickening in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 115:92–96
Chen AL, Shapiro JA, Ahn AK, Zuckerman JD, Cuomo F (2003) Rotator cuff repair in patients with type 1 diabetes mellitus. J Shoulder Elbow Surg 12:416–421
Bruggeman NB, Turner NS, Dahm DL, Voll AE, Hoskin TL, Jacofsky DJ, Haidukewych GJ (2004) Wound complications after open Achilles tendon repair: an analysis of risk factors. Clin Orthop Relat Res 427:63–66
Didomenico LA, Williams K, Petrolla AF (2008) Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Food Ankle Surg 47:463–467
Maffulli N, Longo UG, Maffulli GD, Khanna A, Denaro V (2011) Achilles tendon ruptures in diabetic patients. Arch Orthop Trauma Surg 131:33–38
Chbinou N, Frenette J (2004) Insulin dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. Am J Physiol Regul Integr Comp Physiol 286:R952–R957
Bedi A, Fox AJ, Harris PE, Deng XH, Ying L, Warren RF, Rodeo SA (2010) Diabetes mellitus impairs tendon-bone healing after rotator cuff repair. J Shoulder Elbow Surg 19:978–988
Bayat M, Feridoni MJ, Piryaie A, Dadpay M, Gazour R, Rezaei F, Norouzian M, and Akbari M (2012) Effect of streptozotocin induced type-1 diabetes on tendon healing in rats, a biomechanical and histological study. Int J Diabetes Mellit (in press)
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg 22:141–150
Mester E, Juhasz J, Veraga P, Karika G (1968) Laser in clinical practice. Acta Chir Acad Sci Hung 9:349–357
Enwemeka CS, Rodriguez O, Gall NG, Walsh NE (1990) Morphometric of collagen fibril population in He–Ne laser photostimulated tendons. J Clin Laser Med Surg 8:47–51
Enwemeka CS (1991) Connective tissue plasticity: ultrastructural, biomechanical, and morphometric effects of physical factors on intact and regenerating tendons. J Orthop Sports Phys Ther 14:198–212
Enwemeka CS (1992) Ultrastructural morphometry of membrane-bound intracytoplasmic collagen fibrils in tendon fibroblasts exposed to He–Ne laser beam. Tissue Cell 24:511–523
Reddy GK (2003) Comparison of the photostimulatory effects of visible He–Ne and infrared Ga–As lasers on healing impaired diabetic rat wounds. Lasers Surg Med 33:344–351
Fox AJ, Bedi A, Deng SH, Ying L, Harris PE, Warren RF, Rodeo SA (2011) Diabetes mellitus alters the mechanical properties of the native tendon in an experimental rat model. J Orthop Res 29:880–885
Reddy GK, Stehno-Bittel L, Enwemeka CS (1998) Laser photostimulation of collagen production in healing rabbit Achilles tendon. Lasers Surg Med 22:281–287
Elwakil TF (2007) An in vivo experimental evaluation of He–Ne laser photostimulation in healing Achilles tendons. Laser Med Sci 23:53–59
Carrinho PM, Renno AC, Koeke P, Salate ACB, Parizotto NA, Vidal BC (2006) Comparative study using 685-nm and 830-nm lasers in tissue repair of tenotomized tendons in the mouse. Photomed Laser Surg 24:754–758
Reddy GK, Stenho-Bittel L, Enwemeka CS (2001) Laser photostimulation accelerates wound healing in diabetic rats. Wound Rep Reg 9:248–255
Bayat M, Abdi S, Javadieh F, Mohsenifar ZH, Rashid MR (2009) The effects of low-level laser therapy on bone in diabetic and non diabetic rats. Photomed Laser Surg 27:703–708
Lin JH, Wang MX, Wei A, Zhu W, Diwan AD, Murrell GA (2001) Temporal expression of nitric oxide synthesis isoforms in healing Achilles tendon. J Orthop Res 19:136–142
Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswaldt TM, Beck J, Dorsett-Martin W, Lineaweaver WC (2003) Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg 112:1613–1619
Enwemeka CS (2009) Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg 27:387–393
Yuan J, Murrel GA, Wei AQ, Appleyard RC, DelSoldato P, Wang MX (2003) Addition of nitric oxide via nitroflurbiprofen enhances the material properties of early healing of young rat Achilles tendons. Inflamm Res 52:230–237
Demir H, Menku P, Kirnap M, Calis M, Ikizceli I (2004) Comparison of the effects of laser, ultrasound and combined laser + ultrasound treatments in experimental tendon healing. Lasers Surg Med 35:84–89
Karu TI (1990) Effect of visible radiation on cultured cells. Photochem Photobiol 52:1089–1098
Steinlechner C, Dyson M (1993) The effect of low-level laser therapy on the proliferation of keratinocytes. Laser Ther 5:65–73
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Pires D, Xavier M, Araujo T, Silva JA Jr, Aimbire F, Albertini R (2011) Low-level laser therapy (LLLT, 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Acknowledgments
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei and the Vice-Chancellor of Research at the Medical Faculty of Shaheed Beheshti University MC, Tehran, Iran for financial support (grant no. 13.15183).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nouruzian, M., Alidoust, M., Bayat, M. et al. Effect of low-level laser therapy on healing of tenotomized Achilles tendon in streptozotocin-induced diabetic rats. Lasers Med Sci 28, 399–405 (2013). https://doi.org/10.1007/s10103-012-1074-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1074-7