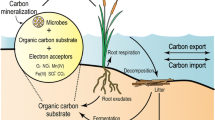
Abstract
Microbial communities inhabiting “subterranean estuaries” along the subsurface freshwater–saltwater continuum determine the fate of nitrogen discharged to coastal waters. Little is known about the microbes that comprise these communities, or what their ecological and biogeochemical responses will be to increased salinity resulting from saltwater intrusion and aquifer salinization. This review covers basic aspects of the nitrogen cycle relevant to the coastal subsurface and provides a framework for predicting the types of microbes and nitrogen transformations that exist in different subterranean estuary systems. Literature concerning the freshwater–saltwater mixing zones of surficial estuaries, where microbial communities are better characterized, is also reviewed to explore what is known about the impact of increasing salinity on both the community composition and biogeochemical function of the microbial assemblage. Collectively, these studies suggest that salinization will alter microbial community composition for all functional groups involved in nitrogen cycling, and may lead to decreases in nitrification and coupled nitrification-denitrification, and increases in dissimilatory nitrate reduction to ammonium (DNRA). Future collaboration between hydrogeologists and microbial ecologists is needed to fully predict the impact of saltwater intrusion on subsurface microbial communities.
Resume
Les populations microbiennes présentes dans les parties souterraines des estuaires au niveau du continuum eau salée - eau douce jouent un rôle dans le devenir de l’azote présent dans les eaux côtières. La connaissance des microbes appartenant à ces populations est peu importante, tout comme les réponses écologiques et biogéochimiques sur l’augmentation de la salinité résultant de l’intrusion saline et de la salinisation des aquifères. Ce bilan des connaissances concerne les aspects fondamentaux du cycle de l’azote dans les zones côtières et fournit un cadre afin de prédire les types de microbes et les transformations de l’azote associées qui existent au niveau des parties souterraines des systèmes estuariens. La littérature concernant les zones de mélange entre les eaux douces et les eaux salées dans les zones de surface des estuaires, où les populations microbiennes sont bien caractérisées, est également passée en revue afin d’analyser la connaissance concernant l’impact de l’augmentation de la salinité, d’une part sur la composition de la communauté microbienne et d’autre part sur la fonction biogéochimique de l’assemblage microbien. De manière générale, ces études suggèrent que la salinisation va modifier la composition de la communauté microbienne pour tous les groupes fonctionnels jouant un rôle dans le cycle de l’azote et qu’elle peut conduire à une réduction de la nitrification et du couple nitrification - dénitrification et à une augmentation de la réduction du nitrate en ammonium (DNRA). Des collaborations dans le futur entre les hydrogéologues et les écologistes microbiens sont nécessaires afin de prédire l’impact des intrusions salines sur les populations microbiennes souterraines.
Resumen
Las comunidades microbianas que habitan los estuarios subterráneos a través del continuo subsuperficial agua dulce - agua salada determinan la subsuelo determinan el destino del nitrógeno descargado en las aguas costeras. Poco es conocido acerca de los microbios que componen estas comunidades, o cuales serán sus respuestas ecológicas y biogeoquímicas al incrementarse la salinidad resultante de la intrusión de agua salada y la salinización del acuífero. Esta revisión cubre aspectos básicos del ciclo del nitrógeno relevantes al subsuelo costero y provee un marco para la predicción de los tipos de microbios y transformaciones del nitrógeno que existen en diferentes sistemas de estuarios subterráneos. También fue revisada la literatura relativa a las zonas del mezcla del agua dulce - agua salada de los estuarios superficiales, donde las comunidades microbianas están mejor caracterizadas, para explorar lo que se conoce acerca del impacto creciente de la salinidad tanto en la composición de la comunidad y como en la función biogeoquímica del conjunto microbiano. Colectivamente, estos estudios sugieren que la salinización altera la composición de la comunidad microbiana para todos los grupos funcionales involucrados en el ciclo del nitrógeno, y puede conducir a reducciones en la nitrificación y en el acople nitrificación - desnitrificación, y aumentos en la disimilitud del nitrato a amonio (DNRA). Se necesita la futura colaboración entre hidrogeólogos y ecólogos microbianos para predecir completamente el impacto de la intrusión de agua salada en las comunidades microbianas subsuperficiales.
摘要
排泄到滨海的N的归宿由咸水-淡水界面连续体“地下河口”处微生物群落决定。但对该群落的微生物组成、生态及其对海水入侵和含水层盐化所导致地下水盐度增加的生物地球化学响应所知甚少。本综述覆盖了关于滨海地下环境中N循环的几个基本方面, 并提供了初步预测不同地下河口系统中可能的微生物种类、N转化过程的方法。本文同时综述了对微生物群落刻画较清楚的地表河口咸水-淡水混合带处的文献, 以探究盐度增加对微生物聚落的群落组成、生物地球化学功能的影响。总体而言, 这些研究显示盐度将改变各种有关N循环的功能群落的微生物群落组成, 可能削弱硝化作用, 及硝化-反硝化的耦合, 此外增加由NO3-还原至NH4+的N。全面预测海水入侵对地下微生物群落的影响, 还需要地质学和微生物生态学者之间的协作。
Resumo
As comunidades microbianas que habitam ‘estuários subterrâneos’ ao longo do continuum subsuperficial água doce-água salgada determinam o destino do azoto descarregado para as águas costeiras. Sabe-se pouco acerca dos micróbios que compõem estas comunidades, ou sobre quais serão as suas respostas ecológicas e biogeoquímicas ao aumento de salinidade devido à intrusão de água salgada e à salinização do aquífero. Esta revisão abrange aspectos básicos do ciclo do azoto, relevantes para a subsuperfície costeira, e fornece um enquadramento para prever os tipos de micróbios e as transformações de azoto que existem em diferentes sistemas estuarinos subterrâneos. A literatura existente relativamente às zonas de mistura de água doce-água salgada de estuários superficiais, onde as comunidades microbianas estão melhor caracterizadas, é também revista, para explorar o que se conhece sobre o impacte do aumento da salinidade quer na composição da comunidade, quer na função biogeoquímica do conjunto microbiano. Em conjunto, estes estudos sugerem que a salinização vai alterar a composição da comunidade microbiana para todos os grupos funcionais envolvidos no ciclo do azoto, e pode conduzir a decréscimos da nitrificação e da nitrificação-denitrificação conjugada, e a aumentos na redução dissimilatória de nitrato para amónia (DNRA). É necessária a colaboração futura de hidrogeólogos e de ecologistas microbianos para predizer completamente o impacte da intrusão de água salgada nas comunidades microbianas subsuperficiais.




Similar content being viewed by others
References
Addy K, Gold A, Nowicki B, McKenna J, Stolt M, Groffman P (2005) Denitrification capacity in a subterranean estuary below a Rhode Island fringing salt marsh. Estuaries 28:896–908
Affourtit J, Zehr JP, Paerl HW (2001) Distribution of nitrogen-fixing microorganisms along the Neuse River Estuary, North Carolina. Microb Ecol 41:114–123
Amann R, Ludwig W (2000) Ribosomal RNA-targeted nucleic acid probes for studies in microbial ecology. FEMS Microbiol Revs 24:555–565
An SM, Gardner WS (2002) Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Mar Ecol Prog Ser 237:41–50
Andersson MGI, Brion N, Middelburg JJ (2006) Comparison of nitrifier activity versus growth in the Scheldt estuary - a turbid, tidal estuary in northern Europe. Aquat Microb Ecol 42:149–158
Berman-Frank I, Lundgren P, Chen YB, Kupper H, Kolber Z, Bergman B, Falkowski P (2001) Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294:1534–1537
Bernhard AE, Donn T, Giblin AE, Stahl DA (2005) Loss of diversity of ammonia-oxidizing bacteria correlates with increasing salinity in an estuary system. Environ Microbiol 7:1289–1297
Bernhard AE, Tucker J, Giblin AE, Stahl DA (2007) Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ Microbiol 9:1439–1447
Boehm AB, Paytan A, Shellenbarger GG, Davis KA (2006) Composition and flux of groundwater from a California beach aquifer: implications for nutrient supply to the surf zone. Continental Shelf Res 26:269–282
Bollmann A, Laanbroek HJ (2002) Influence of oxygen partial pressure and salinity on the community composition of ammonia-oxidizing bacteria in the Schelde estuary. Aquat Microb Ecol 28:239–247
Boudreau BP (2001) Permeable marine sediments: overturning an old paradigm. EOS Trans 82:133–136
Bowen JL, Kroeger KD, Tomasky G, Pabich WJ, Cole ML, Carmichael RH, Valiela I (2007) A review of land-sea coupling by groundwater discharge of nitrogen to New England estuaries: mechanisms and effects. Appl Geochem 22:175–191
Braker G, Tiedje JM (2003) Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl Environ Microbiol 69:3476–3483
Braker G, Fesefeldt A, Witzel KP (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64:3769–3775
Braker G, Zhou JZ, Wu LY, Devol AH, Tiedje JM (2000) Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific northwest marine sediment communities. Appl Environ Microbiol 66:2096–2104
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Caffrey JM, Harrington N, Solem I, Ward BB (2003) Biogeochemical processes in a small California estuary. 2. Nitrification activity, community structure and role in nitrogen budgets. Marine Ecol Prog Ser 248:27–40
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J 1:660–662
Canfield DE, Thamdrup B, Kristensen E (2005) Aquatic Geomicrobiology: Elsevier Academic Press, San Diego
Capone DG, Oneil JM, Zehr JP, Carpenter EJ (1990) Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol 56:3532–3536
Cebron A, Garnier J (2005) Nitrobacter and Nitrospira genera as representatives of nitrite-oxidizing bacteria: Detection, quantification and growth along the lower Seine river (France). Water Res 39:4979–4992
Charette MA (2007) Hydrologic forcing of submarine groundwater discharge: insight from a seasonal study of radium isotopes in a groundwater-dominated salt marsh estuary. Limnol Oceanogr 52:230–239
Charette MA, Sholkovitz ER (2002) Oxidative precipitation of groundwater-derived ferrous iron in the subterranean estuary of a coastal bay. Geophys Res Lett 29. doi:10.1029/2001GL014512
Charette MA, Sholkovitz ER (2006) Trace element cycling in a subterranean estuary: Part 2. Geochemistry of the pore water. Geochim Cosmochim Act 70:811–826
Charette MA, Sholkovitz ER, Hansel CM (2005) Trace element cycling in a subterranean estuary: Part 1. Geochemistry of the permeable sediments. Geochim Cosmochim Act 69:2095–2109
Coci M, Riechmann D, Bodelier PLE, Stefani S, Zwart G, Laanbroek HJ (2006) Effect of salinity on temporal and spatial dynamics of ammonia-oxidizing bacteria from intertidal freshwater sediment. FEMS Microbiol Ecol. 53:359–368
Crump BC, Hopkinson CS, Sogin ML, Hobbie JE (2004) Microbial biogeography along an estuarine salinity gradient: combined influences of bacterial growth and residence time. Appl Environ Microbiol 70:1494–1505
Dale OR, Tobias CR, Song BK (2009) Biogeographical distribution of diverse anaerobic ammonium oxidizing (anammox) bacteria in Cape Fear River Estuary. Environ Microbiol 11:1194–1207
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156:457–464
de Beer D, Wenzhofer F, Ferdelman TG, Boehme SE, Huettel M, van Beusekom JEE et al (2005) Transport and mineralization rates in North Sea sandy intertidal sediments, Sylt-Romo Basin, Wadden Sea. Limnol Oceanogr 50:113–127
de Bie MJM, Speksnijder A, Kowalchuk GA, Schuurman T, Zwart G, Stephen JR et al (2001) Shifts in the dominant populations of ammonia-oxidizing beta-subclass Proteobacteria along the eutrophic Schelde estuary. Aquat Microb Ecol 23:225–236
de la Torre JR, Walker CB, Ingalls AE, Koenneke M, Stahl DA (2008) 12 Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ Microbiol 10:810–818
de Sieyes NR, Yamahara KM, Layton BA, Joyce EH, Boehm AB (2008) Submarine discharge of nutrient-enriched fresh groundwater at Stinson Beach, California is enhanced during neap tides. Limnol Oceanogr 53:1434–1445
Dollhopf SL, Hyun JH, Smith AC, Adams HJ, O'Brien S, Kostka JE (2005) Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Appl Environ Microbiol 71:240–246
Etchebehere C, Tiedje J (2005) Presence of two different active nirS nitrite reductase genes in a denitrifying Thauera sp from a high-nitrate-removal-rate reactor. Appl Environ Microbiol 71:5642–5645
Flanagan DA, Gregory LG, Carter JP, Karakas-Sen A, Richardson DJ, Spiro S (1999) Detection of genes for periplasmic nitrate reductase in nitrate respiring bacteria and in community DNA. FEMS Microbiol Lett 177:263–270
Francis CA, O'Mullan GD, Ward BB (2003) Diversity of ammonia monooxygenase (amoA) genes across environmental gradients in Chesapeake Bay sediments. Geobiology 1:129–140
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
Francis CA, Beman JM, Kuypers MMM (2007) New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J 1:19–27
Freitag TE, Chang L, Prosser JI (2006) Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ Microbiol 8:684–696
Gardner WS, McCarthy MJ, An S, Sobolev D, Sell KS, Brock D (2006) Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol Oceanogr 51:558–568
Grommen R, Dauw L, Verstraete W (2005) Elevated salinity selects for a less diverse ammonia-oxidizing population in aquarium biofilters. FEMS Microbiol Ecol 52:1–11
Hallam SJ, Mincer TJ, Schleper C, Preston CM, Roberts K, Richardson PM, DeLong EF (2006) Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. Plos Biol 4:520–536
Hatzenpichler R, Lebecleva EV, Spieck E, Stoecker K, Richter A, Daims H, Wagner M (2008) A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci USA 105:2134–2139
Hays RL, Ullman WJ (2007a) Dissolved nutrient fluxes through a sandy estuarine beachface (Cape Henlopen, Delaware, USA): Contributions from fresh groundwater discharge, seawater recycling, and diagenesis. Estuaries and Coasts 30:710–724
Hays RL, Ullman WJ (2007b) Direct determination of total and fresh groundwater discharge and nutrient loads from a sandy beachface at low tide (Cape Henlopen, Delaware). Limnol Oceanogr 52:240–247
Head IM, Saunders JR, Pickup RW (1998) Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated microorganisms. Microb Ecol 35:1–21
Herbert RA (1999) Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev 23:563–590
Hopkinson CS, Giblin AE, Tucker J, Garritt RH (1999) Benthic metabolism and nutrient cycling along an estuarine salinity gradient. Estuaries 22:863–881
Howarth RW, Marino R, Lane J, Cole JJ (1988) Nitrogen-fixation in fresh-water, estuarine, and marine ecosystems. 1. Rates and importance. Limnol Oceanogr 33:669–687
Hunter EM, Mills HJ, Kostka JE (2006) Microbial community diversity associated with carbon and nitrogen cycling in permeable shelf sediments. Appl Environ Microbiol 72:5689–5701
Ingalls AE, Shah SR, Hansman RL, Aluwihare LI, Santos GM, Druffel ERM, Pearson A (2006) Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc Natl Acad Sci USA 103:6442–6447
Jetten MSM (2008) The microbial nitrogen cycle. Environ Microbiol 10:2903–2909
Jetten MSM, Cirpus I, Kartal B, van Niftrik L, van de Pas-Schoonen K, Sliekers O et al (2005) 1994–2004: 10 years of research on the anaerobic oxidation of ammonium. Biochem Soc Trans 33:119–123
Joye SB, Hollibaugh JT (1995) influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270:623–625
Karner MB, DeLong EF, Karl DM (2001) Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507–510
Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Ann Rev Microbiol 55:485–529
Kroeger KD, Charette MA (2008) Nitrogen biogeochemistry of submarine groundwater discharge. Limnol Oceanogr 53:1025–1039
Kroeger KD, Swarzenski PW, Greenwood WJ, Reich C (2007) Submarine groundwater discharge to Tampa Bay: nutrient fluxes and biogeochemistry of the coastal aquifer. Mar Chem 104:85–97
Lam P, Jensen MM, Lavik G, McGinnis DF, Muller B, Schubert CJ et al (2007) Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci USA 104:7104–7109
Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D, Gutierrez D, Amann R, Jetten MSM, Kuypers MMM (2009) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Nat Acad Sci USA 106:4752
Laverman AM, Canavan RW, Slomp CP, Van Cappellen P (2007) Potential nitrate removal in a coastal freshwater sediment (Haringvliet Lake, The Netherlands) and response to salinization. Water Res 41:3061–3068
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW et al (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Mackay IM (2004) Real-time PCR in the microbiology laboratory. Clin Microbiol Infect 10:190–212
Magalhaes CM, Joye SB, Moreira RM, Wiebe WJ, Bordalo AA (2005) Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Res 39:1783–1794
Maixner F, Wagner M, Luecker S, Pelletier E, Schmitz-Esser S, Hace K et al. (2008) Environmental genomics reveals a functional chlorite dismutase in the nitrite-oxidizing bacterium ‘Candidatus Nitrospira defluvii’. Environ Microbiol 10:3043–3056
Massey LK, Sokatch JR, Conrad RS (1976) Branched-chain amino acid catabolism in Bacteria. Bacteriol Rev 40:42–54
Meyer RL, Risgaard-Petersen N, Allen DE (2005) Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Appl Environ Microbiol 71:6142–6149
Michael HA, Mulligan AE, Harvey CF (2005) Seasonal oscillations in water exchange between aquifers and the coastal ocean. Nature 436:1145–1148
Mills HJ, Hunter E, Humphrys M, Kerkhof L, McGuinness L, Huettel M, Kostka JE (2008) Characterization of nitrifying, denitrifying, and overall bacterial communities in permeable marine sediments of the northeastern Gulf of Mexico. Appl Environ Microbiol 74:4440–4453
Mohan SB, Schmid M, Jetten M, Cole J (2004) Detection and widespread distribution of the nrfA gene encoding nitrite reduction to ammonia, a short circuit in the biological nitrogen cycle that competes with denitrification. FEMS Microbiol Ecol 49:433–443
Moore WS (1999) The subterranean estuary: a reaction zone of ground water and sea water. Mar Chem 65:111–125
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10:3002–3016
Mulholland MR, Lee C, Glibert PM (2003) Extracellular enzyme activity and uptake of carbon and nitrogen along an estuarine salinity and nutrient gradient. Mar Ecol Prog Ser 258:3–17
Nold SC, Zhou JZ, Devol AH, Tiedje JM (2000) Pacific northwest marine sediments contain ammonia-oxidizing bacteria in the beta subdivision of the Proteobacteria. Appl Environ Microbiol 66:4532–4535
Nowicki BL (1994) The effect of temperature, oxygen, salinity, and nutrient enrichment on estuarine denitrification rates measured with a modified nitrogen gas flux technique. Est Coast Shelf Sci 38:137–156
Ouverney CC, Fuhrman JA (2000) Marine planktonic Archaea take up amino acids. Appl Environ Microbiol 66:4829–4833
Pace NR (1997) A molecular view of microbial diversity and the biosphere. Science 276:734–740
Philippot L (2002) Denitrifying genes in bacterial and Archaeal genomes. Biochimica et Biophysica Acta-Gene Structure and Expression 1577:355–376
Poly F, Wertz S, Brothier E, Degrange V (2008) First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol Ecol 63:132–140
Rappe MS, Giovannoni SJ (2003) The uncultured microbial majority. Annu Rev Microbiol 57:369–394
Rich JJ, Dale OR, Song B, Ward BB (2008) Anaerobic ammonium oxidation (Anammox) in Chesapeake Bay sediments. Microb Ecol 55:311–320
Riesenfeld CS, Schloss PD, Handelsman J (2004) Genomic analysis of microbial communities. Ann Rev Gen 38:525–552
Risgaard-Petersen N, Meyer RL, Schmid M, Jetten MSM, Enrich-Prast A, Rysgaard S, Revsbech NP (2004) Anaerobic ammonium oxidation in an estuarine sediment. Aquat Microb Ecol 36:293–304
Risgaard-Petersen N, Meyer RL, Revsbech NP (2005) Denitrification and anaerobic ammonium oxidation in sediments: effects of microphytobenthos and NO3. Aquat Microb Ecol 40:67–76
Risgaard-Petersen N, Langezaal AM, Ingvardsen S, Schmid MC, Jetten MSM, Op den Camp HJM et al (2006) Evidence for complete denitrification in a benthic foraminifer. Nature 443:93–96
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Rysgaard S, Thastum P, Dalsgaard T, Christensen PB, Sloth NP (1999) Effects of salinity on NH4+ adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22:21–30
Ryther JH, Dunstan WM (1971) Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science 171:1008
Santoro AE, Boehm AB, Francis CA (2006) Denitrifier community composition along a nitrate and salinity gradient in a coastal aquifer. Appl Environ Microbiol 72:2102–2109
Santoro AE, Francis CA, de Sieyes NR, Boehm AB (2008) Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ Microbiol 10:1068–1079
Santos IR, Burnett WC, Chanton J, Mwashote B, Suryaputra IGNA, Dittmar T (2008a) Nutrient biogeochemistry in a Gulf of Mexico subterranean estuary and groundwater-derived fluxes to the coastal ocean. Limnol Oceanogr 53:507–718
Santos IR, Niencheski F, Burnett W, Peterson R, Chanton J, Andrade CFF et al (2008b) Tracing anthropogenically driven groundwater discharge into a coastal lagoon from southern Brazil. J Hydrol 353:275–293
Scala DJ, Kerkhof LJ (1998) Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol Lett 162:61–68
Scala DJ, Kerkhof LJ (2000) Horizontal heterogeneity of denitrifying bacterial communities in marine sediments by terminal restriction fragment length polymorphism analysis. Appl Environ Microbiol 66:1980–1986
Schmid MC, Maas B, Dapena A, de Pas-Schoonen KV, de Vossenberg JV, Kartal B et al (2005) Biomarkers for in situ detection of anaerobic ammonium-oxidizing (anammox) bacteria. Appl Environ Microbiol 71:1677–1684
Schmid MC, Risgaard-Petersen N, van de Vossenberg J, Kuypers MMM, Lavik G, Petersen J, Hulth S, Thamdrup B, Canfield D, Dalsgaard T, Rysgaard S, Sejr MK, Strous M, Op den Camp HJM, Jetten MSM (2007) Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environ Microbiol 9:1476–1484
Scott JT, McCarthy MJ, Gardner WS, Doyle RD (2008) Denitrification, dissimilatory nitrate reduction to ammonium, and nitrogen fixation along a nitrate concentration gradient in a created freshwater wetland. Biogeochemistry 87:99–111
Seitzinger S, Harrison JA, Bohlke JK, Bouwman AF, Lowrance R, Peterson B et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Shimamura M, Nishiyama T, Shigetomo H, Toyomoto T, Kawahara Y, Furukawa K, Fujii T (2007) Isolation of a multiheme protein with features of a hydrazine-oxidizing enzyme from an anaerobic ammonium-oxidizing enrichment culture. Appl Environ Microbiol 73:1065–1072
Short SM, Zehr JP (2007) Nitrogenase gene expression in the Chesapeake Bay Estuary. Environ Microbiol 9:1591–1596
Short SM, Jenkins BD, Zehr JP (2004) Spatial and temporal distribution of two diazotrophic bacteria in the Chesapeake Bay. Appl Environ Microbiol. 70:2186–2192
Slomp CP, Van Cappellen P (2004) Nutrient inputs to the coastal ocean through submarine groundwater discharge: controls and potential impact. J Hydrol 295:64–86
Smith CJ, Nedwell DB, Dong LF, Osborn AM (2007) Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl Environ Microbiol 73:3612–3622
Spiteri C, Slomp CP, Charette MA, Tuncay K, Meile C (2008) Flow and nutrient dynamics in a subterranean estuary (Waquoit Bay, MA, USA): field data and reactive transport modeling. Geochim Cosmochim Act 72:3398–3412
Stephen JR, Chang YJ, Macnaughton SJ, Kowalchuk GA, Leung KT, Flemming CA, White DC (1999) Effect of toxic metals on indigenous soil β-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microbiol 65:95–101
Strous M, Jetten MSM (2004) Anaerobic oxidation of methane and ammonium. Ann Rev Microbiol 58:99–117
Takeuchi J (2006) Habitat segregation of a functional gene encoding nitrate ammonification in estuarine sediments. Geomicrobiol J 23:75–87
Talbot JM, Kroeger KD, Rago A, Allen MC, Charette MA (2003) Nitrogen flux and speciation through the subterranean estuary of Waquoit Bay, Massachusetts. Biol Bull 205:244–245
Taniguchi M (2002) Tidal effects on submarine groundwater discharge into the ocean. Geophys Res Lett 29:1561. doi:10.1029/2002GL014987
Tiedje JM (1988) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Biology of anaerobic microorganisms. Wiley, New York
Tobias CR, Anderson IC, Canuel EA, Macko SA (2001) Nitrogen cycling through a fringing marsh-aquifer ecotone. Mar Ecol Prog Ser 210:25–39
Treusch AH, Leininger S, Kletzin A, Schuster SC, Klenk HP, Schleper C (2005) Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ Microbiol 7:1985–1995
Trimmer M, Nicholls JC, Deflandre B (2003) Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Appl Environ Microbiol 69:6447–6454
Ullman WJ, Chang B, Miller DC, Madsen JA (2003) Groundwater mixing, nutrient diagenesis, and discharges across a sandy beachface, Cape Henlopen, Delaware (USA). Est Coast Shelf Sci 57:539–552
Valiela I, Foreman K, Lamontagne M, Hersh D, Costa J, Peckol P et al (1992) Couplings of watersheds and coastal waters—sources and consequences of nutrient enrichment in Waquoit Bay, Massachusetts. Estuaries 15:443–457
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA et al (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Ward BB, O'Mullan GD (2002) Worldwide distribution of Nitrosococcus oceani, a marine ammonia-oxidizing gamma-proteobacterium, detected by PCR and sequencing of 16 S rRNA and amoA genes. Appl Environ Microbiol 68:4153–4157
Weston NB, Dixon RE, Joye SB (2006) Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J Geophys Res Biogeosci 111. doi:ARTN G01009
Zehr JP, McReynolds LA (1989) Use of degenerate oligonucleotides for amplification of the nifh gene from the marine cyanobacterium Trichodesmium-thiebautii. Appl Environ Microbiol 55:2522–2526
Zehr JP, Ward BB (2002) Nitrogen cycling in the ocean: New perspectives on processes and paradigms. Appl Environ Microbiol 68:1015–1024
Zehr JP, Jenkins BD, Short SM, Steward GF (2003) Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgements
The author gratefully acknowledges Nicholas Nidzieko for assistance in drafting the figures, Nicholas de Sieyes and Kevin Kroeger for helpful discussions, Ed Leadbetter and two anonymous reviewers for valuable input on the manuscript, and the Guest Editors of this special issue. The author was supported by the Postdoctoral Scholar Program at Woods Hole Oceanographic Institution, with funding provided by the United States Geological Survey.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the linked to the electronic supplementary material.
ESM Figure 1
a Locations of studies on the US northeast coast referenced in the text. b Additional US locations referenced in the text (PDF 839 kb)
Rights and permissions
About this article
Cite this article
Santoro, A.E. Microbial nitrogen cycling at the saltwater–freshwater interface. Hydrogeol J 18, 187–202 (2010). https://doi.org/10.1007/s10040-009-0526-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-009-0526-z