Abstract
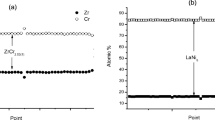
Hydride-forming alloys are used as negative electrode components of nickel metal hydride (MH) batteries. Generally, commercially used compounds are made of two types of alloys, rare earth based on LaNi5, known as AB5 type, and alloys based on ZrCr2, ZrV2, ZrMn2, and TiMn2, known as AB2 type (Laves phases). A and B are generally composed of more than one element. In both systems, the A components are metals that form stable hydrides, while the B components are transition metals and form less stable hydrides. In the present work, electrodes were prepared using composite material obtained by mechanical milling of different proportions of ZrTiV0.8Ni2Cr0.52Mn0.56Co0.08Al0.04 (AB2) and LaNi3.6Co0.7Mn0.4Al0.3 (AB5) alloys. The particles of the AB5 alloy were dispersed on the surface of the AB2 particles, as shown by scanning electron microscopy (SEM) and energy-dispersive spectrometry (EDS). The discharge capacity of the electrodes improved with the addition of 80 and 50 weight-% AB2. The maximum discharge capacities obtained, after 30 cycles, for electrodes with 50 and 80 % AB2 were above 200 mA h g−1, while for the original AB2 alloy it was less than 170 mA h g−1. A decrease in the composite concentration of the AB2 alloy improves the exchange current density, as can be seen from electrochemical impedance spectroscopy (EIS) measurements. High-rate dischargeability (HRD) and EIS results showed enhanced hydrogen diffusion for the samples with an AB2 concentration of 50 and 80 %.









Similar content being viewed by others
References
Ogden JM (1999) Developing an infrastructure for hydrogen vehicles: a Southern California case study. Int J Hydrog Energy 24:709–730
Sakintuna B, Lamaridarkrim F, Hirscher M (2007) Metal hydride materials for solid hydrogen storage: a review. Int J Hydrog Energy 32:1121–1140
Hirscher M (2010) Handbook of hydrogen storage: new materials for future energy storage. doi: 10.1002/9783527629800
Sandrock G (1999) A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J Alloys Compd 293-295:877–888
Ivey DG, Northwood DO (1983) Storing energy in metal hydrides: a review of the physical metallurgy. J Mater Sci 18:321–347
Shaltiel D (1978) Hidride properties of AB2 laves phase compounds. J Less-Common Met 62:407–416
Züttel A (2003) Materials for hydrogen storage. Mater Today 6:24–33
Lasia A (2002) Applications of the electrochemical impedance spectroscopy to hydrogen adsorption, evolution and absorption into metals. In: Conway BE, White RE (eds) Modern aspects of electrochemistry. Springer USA, Berlin Heidelberg New York, pp. 1–49
Prasad Yadav T, Manohar Yadav R, Pratap Singh D (2012) Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci Nanotechnol 2:22–48
Koch CC, Whittenberge JD (1996) Mechanical milling/alloying of intermetallics. Intermetallics 4:339–355
Benjamin JS, Volin TE (1974) The mechanism of mechanical alloying. Metall Trans 5:1929–1934
Wright IG, Wilcox BA (1974) Observations on strengthening and oxidation behavior of a dispersion hardened Fe–Cr–Base alloy prepared by mechanical alloying. Metall Trans 5:957–960
Benjamin JS (1900) Mechanical alloying—a perspective. Met Powder Rep 45:122–127
Zaluski L, Zaluska A, Olsen JOS (1995) Hydrogen absorption in nanocrystalline Mg2Ni formed by mechanical alloying. J Alloys Compd 217:245–249
Yu XB, Walker GS, Grant DM, et al. (2005) Electrochemical hydrogen storage of Ti–V-based body-centered-cubic phase alloy surface-modified with AB5 nanoparticles. Appl Phys Lett 87:133121
Yu XB, Dou T, Wu Z, et al. (2006) Electrochemical hydrogen storage in Ti–V-based alloys surface-modified with carbon nanoparticles. Nanotechnol 17:268–271
Nagai H, Tomizawa H, Ogasawara T, Shoji K-I (1990) Hydriding characteristics of Mg-xwt.%LaNi5 sintered alloys. J Less-Common Met 157:15–24
Jain A, Agarwal S, Vyas D, et al. (2010) Correlation between the milling time and hydrogen storage properties of ZrCrFe ternary alloy. Int J Hydrog Energy 35:9910–9915
Han S-M, Zhao M-S, Zhang Z, et al. (2005) Effect of AB2 alloy addition on the phase structures and electrochemical characteristics of LaNi5 hydride electrode. J Alloys Compd 392:268–273
Han S, Zhang Z, Zhao M, Zheng Y (2006) Electrochemical characteristics and microstructure of Zr0.9Ti0.1Ni1.1Mn0.6V0.3Zr0.9Ti0.1Ni1.1Mn0.6V0.3–LaNi5LaNi5 composite hydrogen storage alloys. Int J Hydrog Energy 31:563–567
Lundqvist A, Lindbergh G (1999) Kinetic study of a porous metal hydride electrode. Electrochim Acta 44:2523–2542
Meyers JP, Doyle M, Darling RM, Newman J (2000) The impedance response of a porous electrode composed of intercalation particles. J Electrochem Soc 147:2930–2940
Paxton B, Newman J (1997) Modeling of nickel/metal hydride batteries. J Electrochem Soc 144:3818–3831
Micka K, Rousar I (1979) Theory of porous electrodes-xvi. The nickel hydroxide electrode. Electrochim Acta 25:1085–1090
Visintin A, Castro E, Real S, et al. (2006) Electrochemical activation and electrocatalytic enhancement of a hydride-forming metal alloy modified with palladium, platinum and nickel. Electrochim Acta 51:3658–3667
Castro E, Real S, Bonesi A, et al. (2004) Electrochemical impedance characterization of porous metal hydride electrodes. Electrochim Acta 49:3879–3890
Humana RM, Thomas JE, Ruiz F, et al. (2012) Electrochemical behavior of metal hydride electrode with different particle size. Int J Hydrog Energy 37:14966–14971
Zaluski L, Zaluska A (1997) Nanocrystalline metal hydrides. J Alloys Compd 254:70–79
Singh A, Singh BK, Davidson DJ, Srivastava ON (2004) Studies on improvement of hydrogen storage capacity of AB5 type:MmNi4.6Fe0.4 alloy. Int J Hydrog Energy 29:1151–1156
Cerón-Hurtado NM, Esquivel MR (2010) Stages of mechanical alloying during the synthesis of Sn-containing AB5-based intermetallics. Int J Hydrog Energy 35:6057–6062
Korea S (1997) The hydrogenation characteristics of Ti-Zr-V-Mn-Ni C14 type Laves phase alloys for metal hydride electrodes. J Alloys Compd 254:601–604
Castro BE, Milocco RH (2005) Identifiability of sorption and diffusion processes using EIS: application to the hydrogen reaction. J Electroanal Chem 579:113–123
Acknowledgments
This work was supported by Agencia Nacional Promoción Científica y Tecnológica of Argentina, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de Catamarca and Universidad Nacional de La Plata.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. B. Castro passed away in 2013
Rights and permissions
About this article
Cite this article
Humana, R.M., Ruiz, F.C., Thomas, J.E. et al. Properties of composites of metal hydride alloys synthesized by mechanical milling. J Solid State Electrochem 21, 153–160 (2017). https://doi.org/10.1007/s10008-016-3347-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3347-8