Abstract
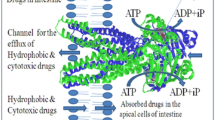
P-glycoprotein (P-gp) has a major role to play in drug pharmacokinetics and pharmacodynamics, since it effluxes many cytotoxic hydrophobic anticancer drugs from gastrointestinal tract, brain, liver and kidney. Piperine is known to enhance the bioavailability of curcumin, as a substrate of P-gp by at least 2000 %. Besides these at least 50 other substrates and inhibitors of P-gp have been reported so far. All P-gp inhibitors have diverse structures. Although little is known about binding of some flavonoids and steroids at the NBD (nucleotide binding domain) of P-gp in the vicinity of ATP binding site inhibiting its hydrolysis, a valid explanation of how P-gp accommodates such a diverse set of inhibitors is still awaited. In the present study, piperine up to 100 μM has not shown observable cytotoxic effect on MDCK cell line, and it has been shown to accumulate rhodamine by fluorescence microscopy and fluorescent activated cell sorter in MDCK cells. Computational simulation for piperine and some first and second generation P-gp inhibitors has shown that these dock at the NBD site of P-gp. A comparative simulation study has been carried out regarding their docking and binding energies. Binding conformation of P-gp co-crystallized complexes with ADP, AMP-PNP (Adenylyl-imidodiphosphate), and ATP were compared with piperine. The receptor based E-pharmacophore of docked piperine has been simulated to find common features amongst P-gp inhibitors. Finally it has been concluded that piperine could be utilized as base molecule for design and development of safe non-toxic inhibitor of P-gp in order to enhance the bioavailability of most of its substrates.

Piperine binds between the consensus sequence of Walker A/P loop and Walker C loop (linker peptide) at the nucleotide binding domain which is crucial for ATP coupled efflux through P-gp. ATP binding competes with piperine. This explains why piperine enhances the bioavailability of its substrate like curcumin by 2000 %







Similar content being viewed by others
References
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PSSR (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta med 64(4):353–357
Bano G, Amla V, Raina RK, Zutshi U, Chopra CL (1987) The effect of piperine on pharmacokinetics of phenytoin in healthy volunteers. Planta Med 53(6):568–569
Zutshi RK, Singh R, Zutshi U, Johri RK, Atal CK (1985) Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J Assoc Phys India 33(3):223–224
Dhanukar S, Kapadia A, Karandikar SM (1983) Influence of trikatu powder on Rifampicin bioavailability. Indian drugs 20:402–404
Loo TW, Clarke DM (1995) Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem 270:843–848
Loo TW, Bartlett MC, Clarke DM (2002) The “LSGGQ” Motif in each nucleotide-binding domain of human P-glycoprotein is adjacent to the opposing Walker—A sequence. J Biol Chem 277(44):41303–41306
Di Pietro A, Dayan G, Conseil G, Steinfels E, Krell T, Trompier D, Baubichon-Cortay H, Jault J (1999) P-glycoprotein-mediated resistance to chemotherapy in cancer cells: using recombinant cytosolic domains to establish structure-function relationships. Braz J Med Biol Res 32(8):925–939
Conseil G, Cortay HB, Guila D, Jault JM, Barron D, Pietro AD (1999) Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci 95:9831–9836
Vishal R, Tandon B, Kapoor G, Bano S, Gupta Z, Gillani S, Gupta DK (2006) P-glycoprotein pharmacological relivance. Indian J Pharmacol 38(1):13–24
Dawson RJP, Locher KP (2006) Structure of bacterial multidrug transporter. Nature 443:180–185
Dawson RJP, Locher KP (2007) Structure of the multidrug ABC transporter Sav 1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett 581:935–941
Ramaen O, Leulliot N, Sizun C, Ulryck N, Pamlard O, Lallemand JY, Tilbeurgh H, Jacquet E (2006) Structure of the human multidrug resistance protein-1 nucleotide binding domain 1 bound to Mg2+/ATP reveals a Non-productive catalytic site. J Mol Biol 359:940–949
Eytan GD, Regev R, Oren G, Hurwitz CD, Assaraf YG (1997) Efficiency of P-glycoprotein- mediated exclusion of rhodamine dyes from multidrug-resistant cells is determined by their passive transmembrane movement rate. Eur J Biochem 248:104–112
Eugida SV, Stromskaya TP, Frolova EA, Stavrovskaya AA (1993) Early steps of P-glycoprotein expression in cell cultures studied with vital flurochrome. FEBS Lett 329:63–66
Christopher JM, Matthew WL, Kim LRB, Gary MP (2001) Pharmacokinetic and pharmacodynamic implications of P-glycoprotein modulation. Pharmacotherapy 21(7):778–796
Goodsell DS, Olson AJ (1990) Automated docking of substrates to proteins by simulated annealing. Protein Struct Funct Gen 8:195–202
Morris GM, Goodsell DS, Huey R, Olson AJ (1996) Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J Comput Aided Mol Des 10:293–304
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8:127–134
McDonald IK, Thornton JM (1994) Satisfying hydrogen bonding potential in proteins. J Mol Biol 238:777–793
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49(11):3315–3321
Dayan G, Jault JM, Baubichon-Cortay H, Baggetto LG, Renoir JM, Baulieu EE, Gros P, Di Pietro A (1997) Binding of steroid modulators to recombinant cytosolic domain from mouse P-glycoprotein in close proximity to the ATP site. Biochemistry 36(49):15208–15215
De Wet H, McIntosh DB, Conseil G, Baubichon-Cortay H, Krell T, Jault JM, Daskiewicz JB, Barron D, Di Pietro A (2001) Sequence requirements of the ATP-binding site within the C-terminal nucleotide-binding domain of mouse P-glycoprotein: structure-activity relationships for flavonoid binding. Biochemistry 40(34):10382–10391
Boumendjel A, Di Pietro A, Dumontet C, Barron D (2002) Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med Res Rev 22(5):512–529
Di Pietro A, Conseil G, Pérez-Victoria JM, Dayan G, Baubichon-Cortay H, Trompier D, Steinfels E, Jault JM, de Wet H, Maitrejean M, Comte G, Boumendjel A, Mariotte AM, Dumontet C, McIntosh DB, Goffeau A, Castanys S, Gamarro F, Barron D (2002) Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell Mol Life Sci 59(2):307–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, D.V., Godbole, M.M. & Misra, K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: simulation for next generation of P-gp inhibitors. J Mol Model 19, 227–238 (2013). https://doi.org/10.1007/s00894-012-1535-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1535-8