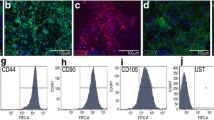
Abstract
While traditional cell culture methods have relied on growing cells as monolayers, three-dimensional (3D) culture systems can provide a convenient in vitro model for the study of complex cell–cell and cell–matrix interactions in the absence of exogenous substrates and may benefit the development of regenerative medicine strategies. In this study, mesenchymal stem cell (MSC) spheroids, or “mesenspheres”, of different sizes, were formed using a forced aggregation technique and maintained in suspension culture for extended periods of time thereafter. Cell proliferation and differentiation potential within mesenspheres and dissociated cells retrieved from spheroids were compared to conventional adherent monolayer cultures. Mesenspheres maintained in growth medium exhibited no evidence of cell necrosis or differentiation, while mesenspheres in differentiation media exhibited differentiation similar to conventional 2D culture methods based on histological markers of osteogenic and adipogenic commitment. Furthermore, when plated onto tissue culture plates, cells that had been cultured within mesenspheres in growth medium recovered morphology typical of cells cultured continuously in adherent monolayers and retained their capacity for multi-lineage differentiation potential. In fact, more robust matrix mineralization and lipid vacuole content were evident in recovered MSCs when compared to monolayers, suggesting enhanced differentiation by cells cultured as 3D spheroids. Thus, this study demonstrates the development of a 3D culture system for mesenchymal stem cells that may circumvent limitations associated with conventional monolayer cultures and enhance the differentiation potential of multipotent cells.







Similar content being viewed by others
References
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Pittenger MF (2008) Mesenchymal stem cells from adult bone marrow. Methods Mol Biol 449:27–44
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Baraniak PR, McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regen Med 5(1):121–143
Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B (2006) Aging of mesenchymal stem cell in vitro. BMC Cell Biol 7:14
Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W (2010) DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell 9(1):54–63
Stolzing A, Coleman N, Scutt A (2006) Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res 9(1):31–35
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3(5):e2213
Ivascu A, Kubbies M (2006) Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen 11(8):922–932
Marrero B, Messina JL, Heller R (2009) Generation of a tumor spheroid in a microgravity environment as a 3D model of melanoma. In Vitro Cell Dev Biol Anim 45(9):523–534
Ong SM, Zhao Z, Arooz T, Zhao D, Zhang S, Du T, Wasser M, van Noort D, Yu H (2010) Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials 31(6):1180–1190
Timmins NE, Nielsen LK (2007) Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med 140:141–151
Kurosawa H (2007) Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng 103(5):389–398
Shukla S, Nair R, Rolle MW, Braun KR, Chan CK, Johnson PY, Wight TN, McDevitt TC (2010) Synthesis and organization of hyaluronan and versican by embryonic stem cells undergoing embryoid body differentiation. J Histochem Cytochem 58(4):345–358
Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC (2009) Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials 30(13):2507–2515
Yang XZ, Kataoka K, Medina R, Yamamoto K, Than SS, Miyazaki M, Huh NH (2009) A novel three-dimensional culture system for isolation and clonal propagation of neural stem cells using a thermo-reversible gelation polymer. Tissue Eng Part C Methods 15(4):615–623
Wan F, Zhang S, Xie R, Gao B, Campos B, Herold-Mende C, Lei T (2010) The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol 20(5):877–889
Ahlenius H, Kokaia Z (2010) Isolation and generation of neurosphere cultures from embryonic and adult mouse brain. Methods Mol Biol 633:241–252
Garzoni LR, Rossi MI, de Barros AP, Guarani V, Keramidas M, Balottin LB, Adesse D, Takiya CM, Manso PP, Otazu IB, Meirelles Mde N, Borojevic R (2009) Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp Cell Res 315(19):3406–3418
Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zund G, Hoerstrup SP (2010) A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol 148(1):46–55
Kelm JM, Djonov V, Ittner LM, Fluri D, Born W, Hoerstrup SP, Fussenegger M (2006) Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng 12(8):2151–2160
Langenbach F, Naujoks C, Kersten-Thiele PV, Berr K, Depprich RA, Kubler NR, Kogler G, Handschel J (2010) Osteogenic differentiation influences stem cell migration out of scaffold-free microspheres. Tissue Eng Part A 16(2):759–766
Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ (2004) Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103(5):1662–1668
Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW (2008) Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One 3(2):e1565
Carpenedo RL, Sargent CY, McDevitt TC (2007) Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cell 25(9):2224–2234
Dennis JE, Carbillet JP, Caplan AI, Charbord P (2002) The STRO-1+ marrow cell population is multipotential. Cell Tissue Organ 170(2–3):73–82
Gregory CA, Gunn WG, Peister A, Prockop DJ (2004) An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 329(1):77–84
Bancroft JD (2002) Theory and practice of histological techniques (5th edn). Churchill Livingstone, Edinburgh
Rim JS, Mynatt RL, Gawronska-Kozak B (2005) Mesenchymal stem cells from the outer ear: a novel adult stem cell model system for the study of adipogenesis. FASEB J 19(9):1205–1207
Huang X, Wang J, Xie H, Zhang Y, Wang W, Yu W, Liu Y, and Ma X (2010) Microcapsules Embedded with Three-Dimensional Fibrous Scaffolds for Cell Culture and Tissue Engineering. Tissue Eng Part C Methods (in press)
Dikovsky D, Bianco-Peled H, Seliktar D (2008) Defining the role of matrix compliance and proteolysis in three-dimensional cell spreading and remodeling. Biophys J 94(7):2914–2925
Ferrante A, Rainaldi G, Indovina P, Indovina PL, Santini MT (2006) Increased cell compaction can augment the resistance of HT-29 human colon adenocarcinoma spheroids to ionizing radiation. Int J Oncol 28(1):111–118
Lin RZ, Chou LF, Chien CC, Chang HY (2006) Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res 324(3):411–422
Robinson EE, Foty RA, Corbett SA (2004) Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell 15(3):973–981
Frith JE, Thomson B, Genever PG (2010) Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. (Translated from eng). Tissue Eng Part C Methods 16(4):735–749
Frisch SM, Screaton RA (2001) Anoikis mechanisms. (Translated from eng). Curr Opin Cell Biol 13(5):555–562
Carpenedo RL, Seaman SA, McDevitt TC (2010) Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation. J Biomed Mater Res Part A 94(2):466–475
Acknowledgments
The authors thank Ms. Martha Lesniewski for help with cell culture and spheroid size analyses, Ms. Sha’Aqua Asberry for assistance with histology sample preparation and Ms. Melissa Kinney for help with image processing. Dr. Baraniak is supported by a Postdoctoral Fellowship from the American Heart Association and this work was supported in part by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Nile Red staining. Following suspension culture under growth or differentiation conditions, mesenspheres were stained with Nile Red and imaged using a confocal microscope to determine the presence of lipid vacuoles. Optical sections through mesenspheres maintained under growth, adipogenic and osteogenic culture conditions demonstrated the presence of lipid vacuoles throughout adipogenic spheroids following 14 days of differentiation. Some lipid vacuole formation was evident in growth spheroids, while none was evident in osteogenic spheroids. Scale bar 20 μm. (JPEG 598 kb)
Supplemental Figure 2
Maintenance of MSC proliferative capacity following suspension culture. Monolayers and MSCs recovered from mesenspheres were plated onto TCPS and stained with BrdU to confirm the presence of proliferating cells within both populations. BrdU staining indicated a population of actively cycling cells within MSCs recovered from mesenspheres following 7 days of suspension culture (bottom panels). The proportion of BrdU+ cells within recovered cell cultures was comparable to that seen in conventional monolayer cultures (top panels). Scale bar 100 μm. (JPEG 641 kb)
Rights and permissions
About this article
Cite this article
Baraniak, P.R., McDevitt, T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res 347, 701–711 (2012). https://doi.org/10.1007/s00441-011-1215-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1215-5