Abstract
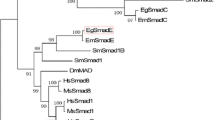
Cyst echinococcosis caused by the matacestodal larvae of Echinococcus granulosus (Eg), is a chronic, worldwide, and severe zoonotic parasitosis. The treatment of cyst echinococcosis is still difficult since surgery cannot fit the needs of all patients, and drugs can lead to serious adverse events as well as resistance. The screen of target proteins interacted with new anti-hydatidosis drugs is urgently needed to meet the prevailing challenges. Here, we analyzed the sequences and structure properties, and constructed a phylogenetic tree by bioinformatics methods. The MIP family signature and Protein kinase C phosphorylation sites were predicted in all nine EgAQPs. α-helix and random coil were the main secondary structures of EgAQPs. The numbers of transmembrane regions were three to six, which indicated that EgAQPs contained multiple hydrophobic regions. A neighbor-joining tree indicated that EgAQPs were divided into two branches, seven EgAQPs formed a clade with AQP1 from human, a “strict” aquaporins, other two EgAQPs formed a clade with AQP9 from human, an aquaglyceroporins. Unfortunately, homology modeling of EgAQPs was aborted. These results provide a foundation for understanding and researches of the biological function of E. granulosus.












Similar content being viewed by others
References
Beitz E (2005) Aquaporins from pathogenic protozoan parasites: structure, function and potential for chemotherapy. Biol Cell 6:373–383
Beitz E, Golldack A, Rothert M, Bülow JV (2015) Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol Ther 155:22–35
Benga G (2012) On the definition, nomenclature and classification of water channel proteins (aquaporins and relatives). Mol Aspects Med 33:514–517
Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T (2009) Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc 4:1–13
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Geadkaew A, Bulow JV, Beitz E, Grams SV, Viyanant V, Grams R (2011) Functional analysis of novel aquaporins from Fasciola gigantaca. Mol Biochem Parastitol 2:144–153
Gowder SM, Chatterjee J, Chaudhuri T, Paul K (2014) Prediction and analysis of surface hydrophobic residues in tertiary structure of proteins. Sci Word J 071258
Hansen M, Kun JF, Schultz JE, Beitz E (2002) A single, bi-functional aquaglyceroporin in blood stage Plasmodium falciparum malaria parasites. J Biol Chem 7:4874–4882
Hemphill A, Stadelmann B, Rufener R, Spiliotis M, Boubaker G, Muller J, Muller N, Gorgas D, Gottstern B (2014) Treatment of echinococcosis: albendazole and menbendazole—what else? Parasite 21:1–9
Kazutoshi T, Yoshinori F (2014) Water channel structures analysed by electron crystallography. Biochim Biophys Acta 1840:1605–1613
Kitchen P, Day RE, Salman MM, Conner MT, Bill RM, Conner AC (2015) Beyond water homeoatasis: diverse functional roles of mammalian aquaporins. Biochimica et Biophysica 12:2410–2421
Perticaroli S, Nickels JD, Ehlers G, Sokolov AP (2014) Rigidity, secondary structure, and the universality of the Boson Peak in proteins. Biophys J 106:2667–2674
Song J (2014) Parasite aquaporins: current developments in drug facilitation and resistance. Biochim Biophys Acta 1840:1566–1573
Song J, He QF (2012) Bioinformatics analysis of the structure and linear B-cell epitopes of aquaporin-3 from Schistosoma japonicum. Asian Pac J Trop Med 2:107–109
Zhang NZ, Huang SY, Zhou DH, Xu Y, He JJ, Zhu XQ (2014) Identification and bioinformatic analysis of a putative calcium-dependent protein kinase (CDPK6) from Toxoplasma gondii. Genet Mol Res 4:10677–10699
Acknowledgments
This study was funded by the National Natural Science Foundation of China (grant numbers 30972567 and 30371258).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Ye, B. Bioinformatics analysis and construction of phylogenetic tree of aquaporins from Echinococcus granulosus . Parasitol Res 115, 3499–3511 (2016). https://doi.org/10.1007/s00436-016-5114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5114-2