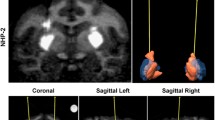
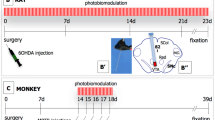
Abstract
We explore the patterns of survival among dopaminergic cells of the midbrain in MPTP-treated macaque monkeys and 6OHDA-lesioned Sprague-Dawley rats. For the monkeys, animals were injected intramuscularly with MPTP for 8 days consecutively and then allowed to survive for 21 days. For the rats, 6OHDA was injected into the midbrain and then allowed to survive for either 7, 28 or 84 days. Brains were processed for tyrosine hydroxylase (TH) and calbindin immunocytochemistry to label populations in the ventral and dorsal tiers of midbrain dopaminergic cells. In monkeys, while there was a decrease in the TH+ cell number in the ventral tier of MPTP-treated cases (65%), there was an overall increase (22%) in the TH+ and calbindin+ cell number in the dorsal tier. Double labelling studies indicate that ∼50% of TH+ cells of the dorsal tier contain calbindin also. In rats, there was a decrease in TH+ cell number in the ventral tier of 6OHDA-lesioned cases (97%), and to a lesser extent, in the TH+ and calbindin+ cell number in the dorsal tier (∼40%). In conclusion, we show a surprising increase in TH+ and calbindin+ cell number in the dorsal tier in response to MPTP insult; such an increase was not evident after 6OHDA insult. We suggest that the increase in antigen expression relates to the dopaminergic reinnervation of the striatum in MPTP-treated cases. We also suggest that the greater loss of dopaminergic cells in the ventral tier when compared to the dorsal tier relates to glutamate toxicity.















Similar content being viewed by others
Abbreviations
- 6OHDA:
-
6 hydroxydopamine
- ABC:
-
Avidin biotin peroxidase complex
- Cb:
-
Calbindin D28 k
- DAB:
-
3,3-diaminobenzidine
- dSNc:
-
Dorsal substantia nigra pars compacta
- GABA:
-
γaminobutyric acid
- III:
-
Oculomotor nerve
- LG:
-
Lateral geniculate nucleus
- MG:
-
Medial geniculate nucleus
- Ml:
-
Medial lemniscus
- MPTP:
-
Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PBS:
-
Phosphate-buffered saline
- R:
-
Red nucleus
- RrF:
-
Retrorubral field
- SC:
-
Superior colliculus
- SNc:
-
Substantia nigra pars compacta
- SNr:
-
Substantia nigra pars reticulata
- Sub:
-
Subthalamus
- TH:
-
Tyrosine hydroxylase
- VL:
-
Ventral lateral nucleus of thalamus
- VP:
-
Ventral posterior nucleus of thalamus
- vSNc:
-
Ventral substantia nigra pars compacta
- VTA:
-
Ventral tegmental area
- ZI:
-
Zona incerta
References
Agid Y, Ruberg M, Javoy-Agid F, Hirsch E, Raisman-Vozari R, Vyas S, Faucheux B, Michel P, Kastner A, Blanchard V (1993) Are dopaminergic neurons selectively vulnerable to Parkinson’s disease? Adv Neurol 60:148–164
Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M (1997) Ataxia and altered dendritic calcium signalling in mice carrying a targeted null mutation of the calbindin D28 k gene. Proc Natl Acad Sci U S A 94:1488–1493
Albanese A, Minciacchi D (1983) Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. J Comp Neurol 216:406–420
Ashkan K, Wallace BA, Mitrofanis J, Brard BY, Fagret D, Benabid AL (2005) MPTP modelling of Parkinson disease: A behavioural, SPECT imaging and immunohistological correlative study. Eur J Neurosci (Submitted)
Augood SJ, Westmore K, McKenna PJ, Emson PC (1993) Co-expression of dopamine transporter mRNA and tyrosine hydroxylase mRNA in ventral mesencephalic neurones. Mol Brain Res 20:328–334
Benazzouz A, Boraud T, Dubedat P, Boireau A, Stutzmann JM, Gross C (1995) Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study. Eur J Pharmacol 284:299–307
Berger B, Trottier S, Gaspar P, Verney C, Alvarez C (1986) Major dopamine innervation of the cortical motor areas in the cynomolgus monkey. A radioautographic study with comparative assessment of serotonergic afferents. Neurosci Lett 72:121–127
Bergman H, Wichmann T, Karmon B, DeLong MR (1994) The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72:507–520
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT (1997) Dopaminergic neurons intrinsic to the primate striatum. J Neurosci 17:6761–6768
Bezard E, Imbert C, Deloire X, Bioulac B, Gross CE (1997) A chronic MPTP model reproducing the slow evolution of Parkinson’s disease: evolution of motor symptoms in the monkey. Brain Res 766:107–112
Bezard E, Boraud T, Bioulac B, Gross CE (1999) Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms. Eur J Neurosci 11:2167–2170
Bezard E, Dovero S, Imbert C, Boraud T, Gross CE (2000) Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. Synapse 38:363–368
Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Björklund A, Hokfelt T (eds) Handbook of chemical neuroanatomy, vol 2. Classical transmitters in the CNS. Elsevier, Amsterdam, pp 55–122
Blanchard V, Raisman-Vozari R, Vyas S, Michel PP, Javoy-Agid F, Uhl G, Agid Y (1994) Differential expression of tyrosine hydroxylase and membrane dopamine transporter genes in subpopulations of dopaminergic neurons of the rat mesencephalon. Brain Res Mol Brain Res 22:29–38
Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R (1996) Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res 709:319–325
Blandini F, Porter RH, Greenamyre JT (1996) Glutamate and Parkinson’s disease. Mol Neurobiol 12:73–94
Braak H, Braak E (1986) Nuclear configuration and neuronal types of the nucleus niger in the brain of the human adult. Hum Neurobiol 5:71–82
Breit S, Bouali-Benazzouz R, Benabid AL, Benazzouz A (2001) Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur J Neurosci 14:1833–1842
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993) The patterns of afferent innervation of the core and shell in the "accumbens" part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338:255–278
Cerruti C, Walther DM, Kuhar MJ, Uhl GR (1993) Dopamine transporter mRNA expression is intense in rat midbrain neurons and modest outside midbrain. Brain Res Mol Brain Res 18:181–186
Christie MJ, Rowe PJ, Beart PM (1986) Effect of excitotoxin lesions in the medical prefrontal cortex on cortical and subcortical catecholamine turnover in the rat. J Neurochem 47:1593–1597
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122:1437–1448
Deutch AY, Goldstein M, Baldino F Jr, Roth RH (1988) Telencephalic projections of the A8 dopamine cell group. Ann N Y Acad Sci 537:27–50
Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ (1991) Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex 1:273–292
van Domburg PH, ten Donkelaar HJ (1991) The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol 121:1–132
Fallon JH, Moore RY (1978) Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol 180:545–580
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114:2283–2301
Fernandez A, de Ceballos ML, Jenner P, Marsden CD (1994) Neurotensin, substance P, delta and mu opioid receptors are decreased in basal ganglia of Parkinson’s disease patients. Neurosci 61:73–79
François C, Yelnik J, Tande D, Agid Y, Hirsch EC (1999) Dopaminergic cell group A8 in the monkey: anatomical organization and projections to the striatum. J Comp Neurol 414:334–347
Gaspar P, Stepneiweska I, Kaas JH (1992) Topography and collateralization of the dopaminergic projection to the motor and lateral prefrontal cortex in owl monkey. J Comp Neurol 325:1–25
Gaspar P, Heizmann CW, Kaas JH (1993) Calbindin D28 k in the dopaminergic mesocortical projection of the monkey (Aotus trivirgatus). Brain Res Bull 603:166–172
Georgievska B, Kirik D, Björklund A (2002) Aberrant sprouting and down regulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting over expression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol 177:461–474
Gerfen CR, Herkenham M, Thibault J (1987a) The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci 7:3915–3934
Gerfen CR, Baimbridge KG, Thibault J (1987b) The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci 7:3935–3944
German DC, Dubach M, Askari S, Speciale SG, Bowden DM (1988) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience 24:161–174
German DC, Manaye K, Smith WK, Woodward DJ, Saper CB (1989) Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol 26:507–514
German DC, Manaye KF, Brown J and Gerfen CR (1990) Calcium binding protein immunoreactivity in the human midbrain: relationship to dopaminergic neurons. Soc Neurosci Abs 16:696; 2919
German DC, Manaye KF, Sonsall PK, Brooks BA (1992) Midbrain, dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28 k-containing cells. Ann NY Acad Sci 648:42–62
German DC, Ng MC, Liang CL, McMahon A, Iacopino AM (1997) Calbindin-D28 k in nerve cell nuclei. Neuroscience 81:735–743
Gibb WR (1992) Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson’s disease. Brain Res 581:283–291
Haber SN, Ryoo H, Cox C, Lu W (1995) Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol 362:400–410
Heise CE, Mitrofanis J (2005) Reduction in parvalbumin expression in the zona incerta after 6OHDA lesion in rats. J Neurocytol (submitted)
Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y (1997) Neuronal vulnerability in Parkinson’s disease. J Neural Transm Suppl 50:79–88
Ho A, Blum M (1998) Induction of interleukin-1 associated with compensatory dopaminergic sprouting in the denervated striatum of young mice: model of aging and neurodegenerative disease. J Neurosci 18:5614–5629
Iacopino A, Christakos S, German D, Sonsalla PK, Altar CA (1992) Calbindin-D28 K-containing neurons in animal models of neurodegeneration: possible protection from excitotoxicity. Mol Brain Res 13:251–261
Jackson A, Crossman AR (1983) Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience 10:725–765
Jennes L, Stumpf WE, Kalivas PW (1982) Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol 210:211–224
Jimenez-Castellanos J, Graybiel AM (1987) Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience 23:223–242
Kalivas PW (1984) Neurotensin in the ventromedial mesencephalon of the rat: anatomical and functional considerations. J Comp Neurol 226:495–507
Kass GE, Wright JM, Nicotera P, Orrenius S (1988) The mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity: role of intracellular calcium. Arch Biochem Biophys 260:789–797
Kirik D, Rosenblad C, Björklund A (1998) Characterization of behavioural and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydomapmine in the rat. Exp Neuro 152:259–277
Kita H, Kitai ST (1987) Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol 260:435–452
Larochelle L, Bedard P, Poirier LJ, Sourkes TL (1971) Correlative neuroanatomical and neuropharmacological study of tremor and catatonia in the monkey. Neuropharmacology 10:273–288
Lavoie B, Parent A (1991) Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport 2:601–604
Lee CS, Sauer H, Björklund A (1996) Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat. Neurosci 72:641–653
Levitt P, Rakic P, Goldman-Rakic P (1984) Region-specific distribution of catecholamine afferents in primate cerebral cortex: a fluorescence histochemical analysis. J Comp Neurol 227:23–36
Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH (1987) The distribution of tyrosine hydroxylase-immunoreactive fibres in primate neocortex is widespread but regionally specific. J Neurosci 7:279–290
Liang CL, Sinton CM, German DC (1996a) Midbrain dopaminergic neurons in the mouse: co-localization with Calbindin-D28 K and calretinin. Neuroscience 75:523–533
Liang CL, Sinton CM, Sonsalla PK, German DC (1996b) Midbrain dopaminergic neurons in the mouse that contain calbindin-D28 k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration 5:313–318
Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC (2004) Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: human midbrain dopamine neurons. J Comp Neurol 473:97–106
Lynd-Balta E, Haber SN (1994) The organization of midbrain projections to the ventral striatum in the primate. Neurosci 59:609–623
Mantz J, Milla C, Glowinski J, Thierry AM (1988) Differential effects of ascending neurons containing dopamine and noradrenaline in the control of spontaneous activity and of evoked responses in the rat prefrontal cortex. Neurosci 27:517–526
McRitchie DA, Halliday GM (1995) Calbindin D28 k-containing neurons are restricted to the medial substantia nigra in humans. Neurosci 65:87–91
McRitchie DA, Cartwright HR, Halliday GM (1997) Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson’s disease. Exp Neurol 144:202–213
Mengod G, Martinez-Mir MI, Vilaro MT, Palacios JM (1989) Localization of the mRNA for the dopamine D2 receptor in the rat brain by in situ hybridization histochemistry. Proc Natl Acad Sci U S A 86:8560–8564
Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR (1989) Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience 32:213–226
Mitsumoto Y, Watanabe A, Mori A, Koga N (1998) Spontaneous regeneration of nigrostriatal dopaminergic neurons in MPTP-treated C57BL/6 mice. Biochem Biophys Res Commun 248:660–663
Mouatt-Prigent A, Agid Y, Hirsch EC (1994) Does the calcium binding protein calretinin protect dopaminergic neurons against degeneration in Parkinson’s disease? Brain Res 668:62–70
Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S (1994) Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol 126:195–204
Ng MC, Iacopino AM, Quintero EM, Marches F, Sonsalla PK, Liang CL, Speciale SG, German DC (1996) The neurotoxin MPTP increases calbindin-D28 k levels in mouse midbrain dopaminergic neurons. Mol Brain Res 36:329–336
Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE (1987) MPTP, MPP+ and mitochondrial function. Life Sci 40:721–729
Ohara PT, Granato A, Moallem TM, Wang BR, Tillet Y, Jasmin L (2003) Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J Neurocytol 32:131–141
Ohye C, Shibazaki T, Hirai T, Wada H, Kawashima Y, Hirato M, Matsumura M (1988) A special role of parvocellular red nucleus in lesion-induced spontaneous tremor in monkeys. Behav Brain Res 28:241–243
Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC (2000) Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience 97:79–88
Palacios JM, Kuhar MJ (1981) Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294:587–589
Parent A, Fortin M, Cote PY, Cicchetti F (1996) Calcium-binding proteins in primate basal ganglia. Neurosci Res 25:309–334
Paxinos G, Watson C (eds) (1986) The rat brain in stereotaxic coordinates 2nd ed. Academic Press, Sydney
Paxinos G, Huang XF, Toga AW (ed) (1998) The Rhesus monkey brain in stereotaxic coordinates. Academic Press, USA
Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, Gaura V, Rascol O, Samson Y, Agid Y (2004) Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol 61:1307–1313
Pelled G, Bergman H, Goelman G (2002) Bilateral overactivation of the sensorimotor cortex in the unilateral rodent model of Parkinson’s disease - a functional magnetic resonance imaging study. Eur J Neurosci 15:389–394
Porrino LJ, Goldman-Rakic PS (1982) Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol 205:63–76
Prensa L, Parent A (2001) The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci 21:7247–7260
Rodriguez M, Gonzalez-Hernandez T (1999) Electrophysiological and morphological evidence for a GABAergic nigrostriatal pathway. J Neurosci 19:4682–4694
Rodriguez M, Barroso-Chinea P, Abdala P, Obeso J, Gonzalez-Hernandez T (2001) Dopamine cell degeneration induced by intraventricular administration of 6-hydroxydopamine in the rat: similarities with cell loss in Parkinson’s disease. Exp Neurol 169:163–181
Sanghera MK, Manaye K, McMahon A, Sonsalla PK, German DC (1997) Dopamine transporter mRNA levels are high in midbrain neurons vulnerable to MPTP. Neuroreport 8:3327–3331
Scheibner T, Törk I (1987) Ventromedial mesencephalic tegmental (VMT) projections to ten functionally different cortical areas in the cat: topography and quantitative analysis. J Comp Neurol 259:247–265
Schneider JS, Yuwiler A, Markham CH (1987) Selective loss of subpopulations of ventral mesencephalic dopaminergic neurons in the monkey following exposure to MPTP. Brain Res 411:144–150
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Seroogy KB, Dangaran K, Lim S, Haycock JW, Fallon JH (1989) Ventral mesencephalic neurons containing both cholecystokinin- and tyrosine hydroxylase-like immunoreactivities project to forebrain regions. J Comp Neurol 279:397–414
Smith Y, Hazrati LN, Parent A (1990) Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. J Comp Neurol 294:306–323
Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ (1998) Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci 18:8003–8015
Steiner H, Kitai ST (2001) Unilateral striatal dopamine depletion: time-dependent effects on cortical function and behavioural correlates. Eur J Neurosci 14:1390–1404
Strick PL, Sterling P (1974) Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J Comp Neurol 153:77–106
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353
Touchon JC, Moore C, Frederickson J, Meshul CK (2004) Lesion of subthalamic or motor thalamic nucleus in 6-hydroxydopamine-treated rats: effects on striatal glutamate and apomorphine-induced contralateral rotations. Synapse 51:287–298
Uhl GR, Goodman RR, Snyder SH (1979) Neurotensin-containing cell bodies, fibres and nerve terminals in the brain stem of the rat: immunohistochemical mapping. Brain Res 167:77–91
Vandermaelen CP, Kocsis JD, Kitai ST (1978) Caudate afferents from the retrorubral nucleus and other midbrain areas in the cat. Brain Res Bull 3:639–644
Wallace BA, Ashkan K, Mitrofanis J, Brard BY, Fagret D, Benabid AL (2005) The protective effect of subthalamotomy on nigral degeneration in MPTP-treated primates. (in preparation)
Waters CM, Peck R, Rossor M, Reynolds GP, Hunt SP (1988) Immunocytochemical studies on the basal ganglia and substantia nigra in Parkinson’s disease and Huntington’s chorea. Neurosci 25:419–438
Yamada T, McGeer PL, Baimbridge KL, McGeer EG (1990) Relative sparing in Parkinson’s disease of substantia nigra dopamine neurons containing calbindin-D28 K. Brain Res 526:303–307
Acknowledgements
We thank Medtronic and Tenix corp/Salteri family for their most generous funding of this work. Laurance Grotti and Sharon Spana provided invaluable technical assistance with the immunostaining and histology, while Rolande Gerbex and Louis Gonzales provided excellent assistance looking after the animal house.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fitzpatrick, E., Ashkan, K., Wallace, B.A. et al. Differential survival patterns among midbrain dopaminergic cells of MPTP-treated monkeys and 6OHDA-lesioned rats. Anat Embryol 210, 101–123 (2005). https://doi.org/10.1007/s00429-005-0003-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-005-0003-y